What is Quality Assurance?
Quality assurance (QA) is a systematic approach utilized by various industries to make sure that pre-determined quality requirements are met and fulfilled. It is composed of principles, methods, protocols, and procedures established and maintained by a company to ensure that the quality of its products and services is consistently high and compliant with organizational and regulatory standards. QA assists workers in meeting quality objectives for each product or service rendered.
What is Quality Control?
Quality control (QC) is a methodical procedure used to ensure that a product or service meets specified requirements and standards. It involves systematic actions and measures to verify that a product or service being developed meets a business’s predefined criteria for quality.
Quality Assurance vs Quality Control
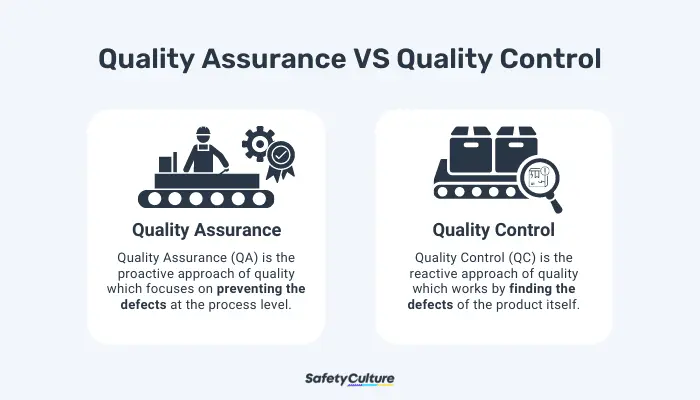
Whereas quality assurance is focused on the processes involved in producing the output, quality control (QC) is more on the inspection of the quality of the products. This is performed by evaluating if they pass specific quality standards before being shipped out to customers. As both parts of the bigger umbrella of quality management, QC aims to catch deficiencies in quality while QA aims to prevent those deficiencies from even occurring.
Quality Assurance and Quality Control | Start with this Basic QA Inspection Checklist
The Importance of Both
Good quality assurance and quality control are two of the most important elements of a successful operation in various industries, such as manufacturing and engineering. Achieving, ensuring, and maintaining the quality of your goods and services are paramount to making your customers happy and keep them coming back.
“Quality is everyone’s responsibility.” – W. Edwards Deming, engineer, statistician, professor, and author
Create Your Own Quality Assurance & Quality Control Template
Eliminate manual tasks and streamline your operations.
Get started for FREEQuality in Manufacturing
This can serve as a beginner’s introduction to the common ISO standards in use today, especially in the industry of manufacturing.
What is ISO and Why is it Important?
ISO is the International Organization for Standardization, with a current membership of 165 countries or national standards bodies. It is the world’s leading authority and regulatory body for safety and quality standards in manufacturing and service.
What are ISO Standards?
ISO standards are specific criteria set by ISO to cover different aspects of business and manufacturing operations. To earn a coveted ISO standard certification, a company has to request and pass a third-party ISO audit performed by a national or regional certification body.
Common ISO Standards
To date, ISO boasts almost 22,000 standards and certifications covering a wide variety of operational aspects. Below are some of the most common standards companies strive for:
ISO 9001:2015 – Quality Management Standard
Last updated in 2015, ISO 9001:2015 specifies the requirements for a company’s QMS (Quality Management System). It aims to assess an operation’s ability to consistently deliver high-quality products and services while meeting customer expectations and relevant statutory and regulatory requirements.
ISO 22000 – Food Safety Standard
ISO 22000 provides requirements for developing and implementing a Food Safety Management System (FSMS). This certification is a must-have for companies in the food processing and food service industries.
ISO 45001:2018 – Workplace Safety Standard
Last updated in 2018, ISO 45001 sets the international standard for Occupational Health & Safety (OH&S) Management Systems. Failure to comply can lead to incidents, cost overruns, and even lawsuits.
Other notable ISO standards include ISO 14001 (Environmental Management), ISO 17025 (Laboratory Testing), and ISO 13485 (Quality Management of Medical Devices).
Quality Control Procedures
Here are some general prerequisites to establish and execute quality control procedures efficiently:
Build a Dedicated Quality Team
Having a dedicated QA/QC Team composed of personnel with the proper credentials should be a priority. Before creating a team, consider the employee’s knowledge of your specific product/service along with prior experience in the same industry before they joined your company.
Define Your Performance Goal
Quality teams must consider the current performance of their product and/or service, define a SMART goal, and then strive to eliminate all of the processes, steps, and obstacles that do not contribute to the achievement of said goal.
Choose the Best Method
Aside from the ones mentioned previously in the article, there are many other quality assurance and quality control methods available today. While Six Sigma, Lean, and Kaizen offer generally applicable procedures for improving overall quality, using the right tools to implement these methods increases their effectiveness.
How to Improve QC Procedures
Consistently delivering high-quality products through processes that are at par with industry standards and looking out for ways to further improve quality control can help your business stay on top.
Step 1: Engage all staff
Involve all employees and gain their buy-in and commitment to adhere to the organization’s quality standards and processes. Conduct onboarding for new hires and refresher quality assurance training for tenured employees to help them understand that maintaining the quality of products and services is everyone’s responsibility.
Step 2: Be proactive
Reinforce quality control procedures through regular quality checks and immediately address nonconformance discovered during internal audits. Implement production planning and control or production control techniques to further strengthen the quality control procedures.
Step 3: Automate recordkeeping
Utilize automated digital recordkeeping to save time and ensure that internal checks are consistently recorded.
Step 4: Seek improvement opportunities
Conduct customer satisfaction surveys, review incident reports, learn from past mistakes in your organization and others in the business, and stay up-to-date with industry best practices.
Step 5: Utilize technology
Save time and maximize resources for quality control by using new technology such as sensors that automate monitoring of quality parameters and mobile auditing tools to record and analyze information that paper audit forms cannot capture.
The Pros and Cons of Internal vs. External QA Practices
Regardless of your QA method of choice, each has its own pros and cons.
Pros and Cons of Internal QA Inspections
| Pros | Cons |
| No need to hire external consultants and auditors so the company saves money. | The data collected may be biased and may be used to cover up employee negligence and other malicious activities. |
| Since actual employees of the company are performing the audit, they are sure to have good context and a proper understanding of what quality looks like for the business. | Management may end up becoming too lenient when it comes to enforcing recommended changes since there is no external pressure to do so. |
| The internal audit department can verify the accuracy of inspection reports faster since data is available in-house. | Internal QA inspectors may become more and more complacent over time as they start treating the practice like a chore. |
Pros and Cons of External QA Inspections
| Pros | Cons |
| Third-party quality inspectors bring additional experience and knowledge from conducting quality inspections for several different companies. | Hiring a third-party quality inspector to conduct QA audits can be expensive. |
| Third-party inspectors can provide an unbiased and objective assessment report. | QA inspections may end up becoming ineffective if they lack the specialized knowledge required to understand and properly assess a specific product/service. |
FAQs about Quality Assurance and Quality Control
In the context of quality management, QA comes first because it involves establishing processes and systems to prevent defects from occurring, while QC comes after to detect and correct defects that may have occurred despite the QA efforts.
Built by a project team and usually based on PDCA technique, a quality assurance plan is a document that provides a guideline with the intent to produce products or services that meet customer requirements and expectations.
A QCP is a document that provides processes and defines milestones in production where products or services are to be inspected to ensure that they are meeting specifications.
While both aim to catch deficiencies in quality, QAP intends to lay down processes aiming to produce output that performs as expected while QCP aims to catch deficiencies in output usually through inspections.
- QAP intends to prevent defects in output.
- QCP catches defects in output.
Some common examples of Quality Assurance processes include:
For Quality Control:
Quality management involves the comprehensive planning, execution, and monitoring of processes to ensure that products or services meet predefined standards. It encompasses quality assurance, which specifically focuses on preventing defects by establishing systematic processes and guidelines.




