What are Quality Standards?
Quality standards refer to sets of guidelines, systems, methods, requirements, and specifications followed by an organization to ensure consistent process and product quality. Mostly prevalent in manufacturing, quality standards are established by industry regulatory boards to help drive customer satisfaction and maintain compliance. Globally, ISO quality standards are the most widely accepted set of standards applicable to various industries.
Why are Quality Standards Important?
Any product or service that’s free from any manufacturing defect, deficiency, or process variation is considered of good quality. This is achieved through the holistic process of quality assurance and quality control.
Thus, to ensure and maintain good manufacturing practices, a set of manufacturing quality standards must be established and followed for the purpose of uniformity across the entire manufacturing process.
Additionally, some of the benefits of quality standards include the following:
- Continuous improvement of quality outcomes
- Efficient adherence to regulatory requirements and compliance
- Reduced process variation and product defects
- Improved worker productivity and safety
- Enhanced customer satisfaction
- Seamless flow of operations
Principles
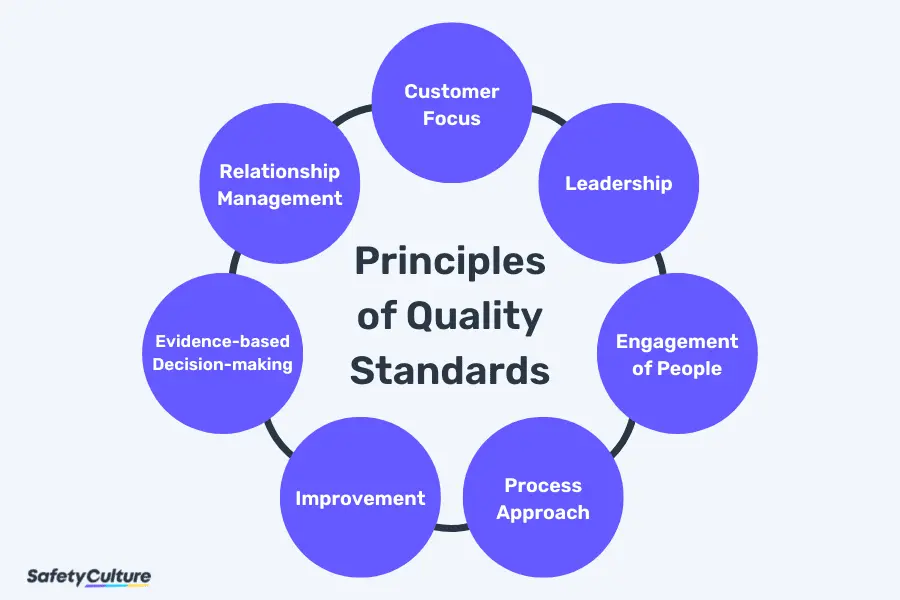
Principles of Quality Standards
In setting quality assurance standards, the 7 principles of quality management also apply to help establish a more fitting Quality Management Standard (QMS) based on your business and industry. That said, the principles of quality standards are the following:
- Customer Focus – This guides the organization in ensuring that customer needs and expectations are met by setting quality standards aligned with them.
- Leadership – This pertains to the organization’s commitment to implement leadership principles across the organization to promote a healthy culture of collaboration.
- Engagement of People – This is key to maintaining worker engagement toward providing better value to the organization and customers.
- Process Approach – This refers to the way of treating all projects and processes as part of a holistic functioning system to drive all efforts into sustaining the business.
- Improvement – This emphasizes the need for an organization to continuously improve by encouraging proactive innovation and consistent recognition of successful initiatives.
- Evidence-based Decision-making – This empowers people to value the importance of data and analysis and how they can put them into practical applications such as maintaining quality standards.
- Relationship Management – This enables organizations to look at the overall supply chain management and how it affects the processes, stakeholders, and suppliers.
What are Examples of Quality Standards?
Learn more about this list of quality standards most widely used in the manufacturing, healthcare, food, and automotive industries.
ISO 9000 and 9001
The International Organization for Standardization (ISO) published the ISO 9000, the family of good management practices standards for QMS. The ISO sets quality control standards for manufacturing companies to adhere to so that they can maintain compliance with industry standards and regulatory requirements. Under ISO 9000 is ISO 9001, which sets out detailed requirements for establishing a QMS that specifically suits their industry needs.
Create Your Own QMS Audit Checklist
Eliminate manual tasks and streamline your operations.
Get started for FREEGMP and GLP
Another set of notable quality standards examples is Good Manufacturing Practices (GMP) and Good Laboratory Practice (GLP).
GMP refers to the system of processes and documentation to ensure that manufacturing products are being produced and controlled consistently against quality standards. On the other hand, GLP is a set of principles that ensures non-clinical laboratory studies are following quality standards and maintaining the integrity of safety test data.
US FDA
Both the GMP and GLP are enforced by the US Food and Drug Administration (FDA). That said, the US FDA sets out regulations that address manufacturing practices, including personal hygienic practices, facility maintenance guidelines, sanitary operations, and process controls during food manufacturing.
IATF 16949
The International Automotive Task Force (IATF) 16949 was created specifically for the automotive industry. Apart from helping organizations maintain the quality of automotive services and assembly parts, IATF 16949 also guides in continuously improving how manufacturers carry out their processes toward reducing defects and waste.
IAQG 9100
The International Aerospace Quality Group (IAQG) 9100 is a specific set of QMS requirements for aviation, space, and defense organizations in the aerospace industry. This can be applied to all supply chain levels to achieve optimal quality and efficiency.




