What is an FDA Inspection?
The US Food and Drug Administration (FDA) inspects facilities to ensure compliance with laws and regulations surrounding the manufacturing, labeling, and handling of food, drugs, medical devices, and cosmetics that are commercially distributed in the US. FDA inspections are conducted on both local and foreign establishments as long as the products are intended for US distribution.
Types of Food and Drug Administration Inspections
The FDA prioritizes the inspection of companies that manufacture high-risk drugs and equipment. Newly registered establishments and those that have past violations are also given priority for FDA audits. Here are four types of FDA inspections that manufacturers can expect:
- Pre-approval – This type of inspection is conducted when there’s an application to market a new product.
- Routine – This is a standard inspection conducted once every two years for domestic establishments and around once every nine years for foreign facilities. If violations are found but corrective actions are immediately taken to address the issues while the FDA inspector is present, this will be noted as a positive indicator of the establishment’s commitment to stay compliant.
- “For-cause” – This inspection is intended to investigate issues or concerns that have been brought to the FDA’s attention.
- Compliance follow-up – If violations are found during an inspection, an FDA Form 483 will list them. Then, the FDA will return to reinspect to confirm if corrective actions were taken to rectify the issues previously observed.
What is an FDA Inspection Checklist?
An FDA inspection checklist, also known as an FDA audit checklist or an inspection preparedness checklist, is a valuable tool used to prepare for FDA inspections. It consists of items and areas that FDA inspectors typically look for during their visits. This tool enables you to perform and document internal audits efficiently, right in time for an FDA inspection.
Why is it Necessary to Have a Checklist for Inspection Readiness?
The primary goal of an FDA inspection is to protect consumer well-being by ensuring that only safe-to-use products are released to the market. In connection with this, new products require FDA approval before they are introduced to the US market, which is why readiness for FDA inspections should be a priority for companies.
An FDA inspection preparedness checklist is a vital component of inspection readiness for various reasons. For instance, it provides a concise overview of the things required during FDA audits. This way, you can adequately prepare for anything an inspector might ask when they visit your facility. Moreover, it also allows you to:
- Determine how compliant your facility is with FDA laws and standards, such as Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP)
- Anticipate potential problems during the inspection and resolve them early on
- Document protocol changes and corrective actions implemented
- Organize all necessary documents for ease of handover and review
- Comply with FDA regulations and quality standards
What to Include in an Inspection Preparedness Checklist
Now that you know the importance of having a checklist when preparing for FDA inspections, you might be wondering how to make one for your internal audits. Here are 7 things that your FDA inspection preparedness checklist should consist of:
- General Information – Begin your checklist with basic details, such as the facility to be inspected, the date of inspection, the name of the officer in charge, and the inspection location.
- Administrative – Ensure proper information dissemination among relevant staff members and a review of Standard Operating Procedures (SOP) for inspections, employee schedules, and clinical equipment.
- Regulatory – Check if all regulatory documents for FDA inspections, such as protocols, logs, disclosure forms, site SOPs, correspondences, and so forth, are complete.
- Clinical – Evaluate existing clinical practices, protocols, reports, and other necessary documentation.
- Pharmacy – Review pharmacy-related documents for completeness and accuracy, such as staff records, licenses, FDA Form 1572, participant profiles, receipts, logs, and SOPs.
- Laboratory – Look into existing laboratory records, including certifications, laboratory results, quality control data, corrective action reports, temperature logs, and so forth.
- Sign-Off – Note your comments and observations during the inspection and affix your signature as proof of FDA audit completion.
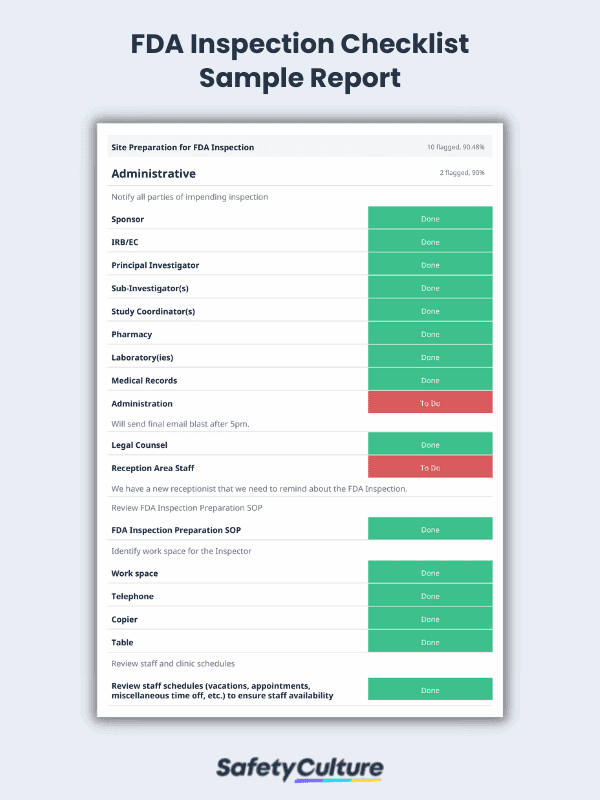
To better illustrate this, here’s a sample FDA inspection report when completed using a digital platform:
FAQs about FDA Inspections
Following the FDA Food Safety Modernization Act and the Federal Food, Drug, and Cosmetic (FD&C) Act, the frequency of FDA inspections will vary depending on the risk level of a facility. High-risk facilities will be inspected at least once every 3 years, while non-high-risk ones will be checked at least once every 5 years.
The duration of FDA inspections will depend on the following factors:
- Business size
- Type of business operations
- Number, type, and complexity of the issues found
- Review of documentation and procedures
Yes, the FDA can perform inspections without any notice in advance for for-cause inspections. Otherwise, such as for routine inspections, they will usually announce inspections around 1-14 days ahead of time.