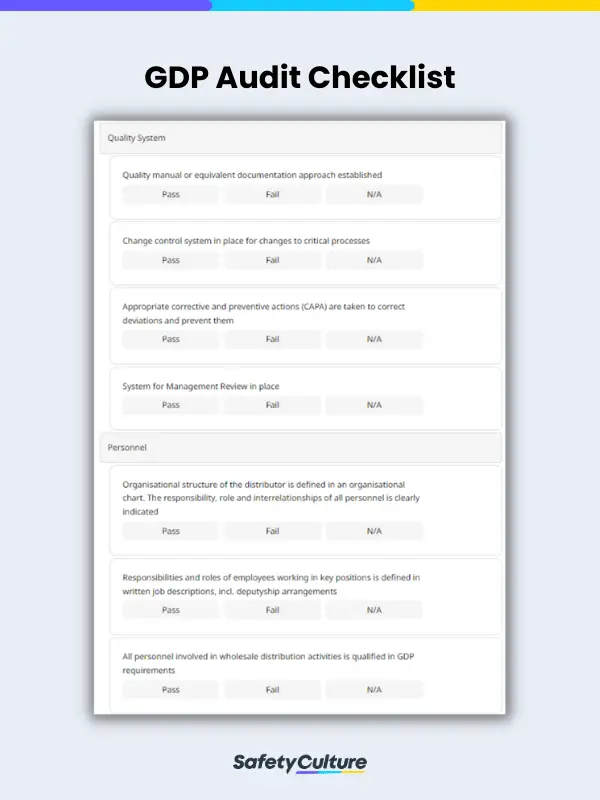
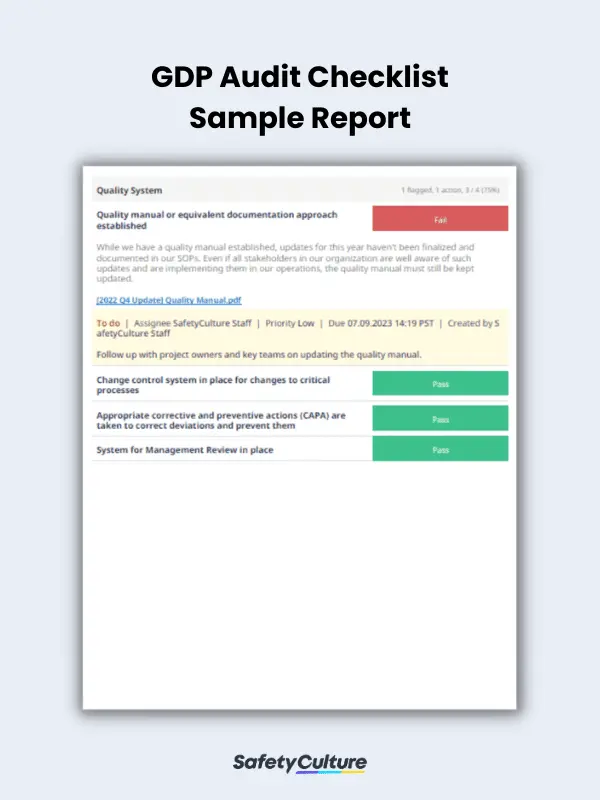
What is a GDP Audit Checklist?
A GDP audit checklist is a structured and comprehensive tool used by organizations to assess and ensure adherence to established standards and guidelines for the proper distribution of products, such as medicines. This tool is designed to help inspectors and auditors systematically evaluate various aspects of distribution processes, facilities, and practices to guarantee the quality, safety, and integrity of products as they move through the supply chain.
Why Use a GDP Audit Checklist
GDP acts as a safeguard for product quality and safety during distribution. It ensures that products maintain their intended condition, reducing risks of contamination, degradation, or damage. As a result, companies can uphold product integrity, reduce recalls, and promote transparency in distribution processes, ultimately contributing to consumer safety, brand reputation, and regulatory adherence. These are just some reasons why it’s important for organizations to maintain compliance with GDP.
To help them verify their GDP compliance, audits and inspections must be conducted using GDP audit checklists. These serve as essential tools for both internal evaluations and external audits to assist organizations in ensuring safe and quality product handling, storage, and transportation.
By integrating a GDP audit checklist into their best practices, companies can not only meet regulatory obligations but also minimize risks, optimize their distribution operations, and achieve the following benefits:
- Compliance Assurance – provides a structured framework to ensure compliance with legal standards and guidelines governing the distribution of products
- Process Optimization – serves as a tool for identifying inefficiencies, bottlenecks, or areas for enhancement in their distribution practices, leading to increased operational efficiency
- Documentation and Accountability – provides documented evidence of compliance efforts
- Cross-Functional Alignment – encourages collaboration among various departments involved in the distribution process
- Preparedness for Audits – simplifies the audit process by ensuring that all necessary criteria are met and documented, leading to smoother audits and reduced stress
Key Components of a GDP Audit Compliance Checklist
The sections, items, and areas covered in a GDP checklist span various aspects of distribution operations and practices. Here are the most essential elements to include to make it comprehensive:
- Quality System
- Personnel
- Premises and Equipment
- Outsourcing Activities
- Layout of Premises
- Hygiene
- Temperature and Environment Control
- Computer Systems
- Documentation
- Operations
- Supplier Qualification
- Qualification of Customers
- Receipt of Goods
- Storage
- Segregation of Goods
- Destruction of Obsolete Goods
- Picking and Packing
- Export (exceptions)
- Complaints, Returns, and Recalls
- Transportation
Guidelines for Creating One
Conducting an internal GDP audit helps organizations prepare for the external audit, usually accomplished by third-party institutions or agencies. To help guide them during this preparation, creating their own checklists is encouraged.
Here’s a step-by-step process on how to make one:
- Align your checklist with relevant regulatory guidelines and standards that govern your industry’s distribution practices and break down the distribution process into key areas.
- Engage professionals with expertise in distribution, quality assurance, and compliance to provide insights and input during the checklist development process.
- Involve representatives from various departments to ensure that the checklist covers all aspects of distribution.
- Integrate risk assessment into the checklist to identify potential risks associated with each aspect of distribution and incorporate risk mitigation strategies.
- Consider using digital tools or software to create, manage, and update the checklist to enhance collaboration and streamline the auditing process.
How to Conduct a GDP Audit Using a Checklist
Keep the following steps and tips in mind when performing a GDP audit with the help of a checklist:
- Form an audit team comprising individuals with expertise in distribution, quality assurance, compliance, and relevant departments.
- Set a date and time for the audit and notify the relevant teams to ensure their availability.
- Provide an overview of the audit process and familiarize the team with the checklist, ensuring they understand the criteria for each item.
- Begin the audit proper by following the checklist systematically. Document observations, findings, and evidence for each item by using notes and photos.
- Collect supporting evidence to substantiate compliance or non-compliance with each item. This can include records, logs, and other documentation.
- Identify areas of non-compliance and recommend corrective actions to address deficiencies.
- Prepare a comprehensive report that includes all key findings and results of the conducted audit. Present it to relevant stakeholders and work with responsible teams to implement the recommended corrective actions as needed.
- Conduct a follow-up audit to assess the implementation and effectiveness of corrective actions.
Training on GDP Audit Checklist Implementation
Training your employees on the effective use of GDP audit checklists is crucial to ensure that they understand its purpose and can contribute to maintaining high-quality distribution practices.
For a comprehensive training program, here are a few of the most important topics and items to be covered:
Importance
- Emphasize the significance of GDP compliance and the role of the checklist.
- Highlight the benefits of effective checklist implementation for the organization.
Contents
- Break down the key components and sections of the GDP audit checklist.
- Explain the requirements associated with each item and walk participants through the structure of the checklist.
- Provide context by referring to relevant regulatory standards and industry guidelines.
- Clarify any technical terms or industry jargon used.
GDP Audit Preparation
- Ensure that participants can understand and interpret the checklist easily.
- Describe how to prepare for an upcoming GDP audit using the checklist.
Documentation and Recordkeeping
- Highlight the importance of gathering documentation and evidence in advance.
- Explain the steps to conduct an audit, including how to accurately document observations, findings, and evidence.
- Discuss the format and structure of the audit report.
Practical Examples and Use Cases
- Present real-world scenarios related to distribution challenges and ask participants to identify checklist items that address those challenges.
- Share examples of successful checklist implementation leading to improved distribution practices.
- Discuss instances where non-compliance with the checklist resulted in issues.
Mock GDP Audits and Performance Feedback
- Provide feedback on participants’ performance during mock audits.
- Encourage open discussions on best practices and areas for improvement.
- Stress the importance of collaboration between departments to ensure checklist accuracy and completeness.
- Highlight how effective communication enhances the audit process and overall distribution practices.
- Encourage participants to provide feedback on the checklist’s usability and effectiveness.
- Discuss the role of feedback in evolving and improving the checklist over time.
- Allocate time for participants to ask questions and seek clarifications on any aspects of the GDP audit checklist or its implementation.
Debriefing
- Summarize the key points covered during the training.
- Reinforce the role of participants in upholding quality distribution practices through checklist implementation.
FAQs About GDP Audit Checklists
By identifying areas for enhancement and addressing non-compliance, a GDP audit checklist drives a culture of continuous improvement in organizations. One example is that feedback from audits conducted with the help of checklists informs strategies to optimize distribution processes over time.
Yes, a GDP audit checklist can be customized. Requirements and challenges can vary significantly based on the nature of the products being handled by an organization and the specific industry in which the distribution takes place. Hence, customization ensures that the checklist is relevant, effective, and aligned with the unique needs of each sector.
For instance, here’s a sample pharmaceutical audit checklist that organizations can use in inspecting and verifying the effectiveness of existing quality and safety systems on pharmaceutical processes.
GDP (Good Distribution Practices) focuses on the proper storage, transportation, and distribution of finished products. GMP (Good Manufacturing Practices), on the other hand, puts emphasis on the production and manufacturing of goods, ensuring consistent quality and safety during the process.
Despite their unique functions and purpose, both are quality assurance systems designed to ensure the safety, quality, and integrity of products in different stages of the supply chain.
For inspecting GMP practices in an organization, using a GMP audit checklist is recommended.