Published 26 Sept 2025
Article by
4 min read
What is ISO 13485:2016?
ISO 13485:2016 is an international standard developed by the International Organization for Standardization (ISO) for the Quality Management System (QMS) of organizations involved in the manufacturing, distribution, servicing, and disposal of medical devices. Organizations with ISO 13485:2016 certification are recognized to produce medical devices that are at par with industry standards.
What is an ISO 13485:2016 Audit Checklist?
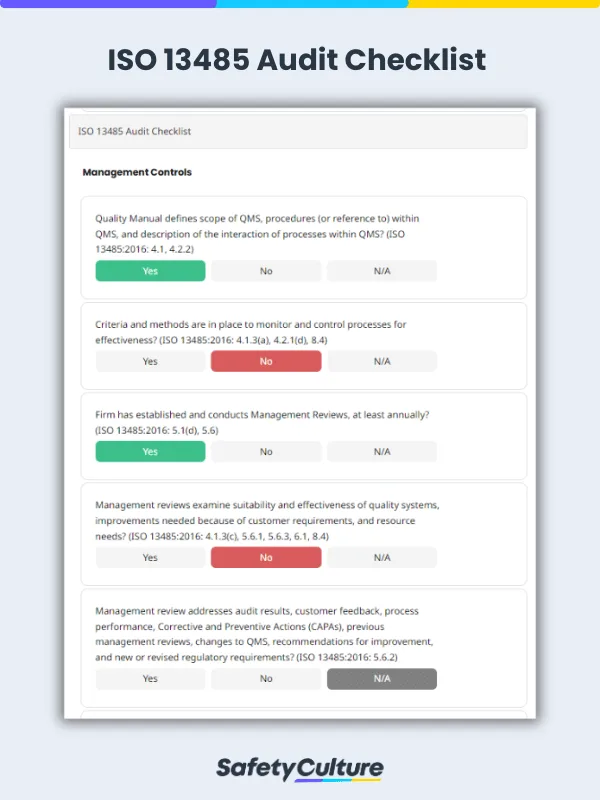
An ISO 13485:2016 audit checklist is a tool used by quality managers to determine if the organizations’ QMS for medical devices aligns with the requirements of the ISO 13485:2016 standard. The checklist includes a comprehensive list of questions, criteria, or statements that are useful in evaluating readiness for a third-party ISO 13485:2016 certification audit.
Why is ISO 13485 Audits Important for your Organization
ISO 13485 audit checklists play a crucial role in ensuring the effectiveness and compliance of an organization’s QMS for medical devices, as well as its process of auditing. Apart from that, here are more key reasons why using these checklists is important:
Helps auditors conduct a thorough evaluation of the QMS
Verifies compliance with the standard’s requirements
Promotes standardization and consistency across audits
Assists auditors to identify areas of non-compliance, gaps, and areas for continuous improvement within the organization’s current practices
Ensures proper documentation and evidence collection during audits
Prepares organizations for external audits and certification assessments
What Should be Included in an ISO 13485 Audit Checklist?
An ISO 13485 audit checklist should cover various aspects of a quality management system (QMS) for medical devices to ensure compliance with the ISO 13485 standard. While the specific items on the checklist may vary based on the organization’s unique processes and activities, here are key areas that should typically be included:
Title Page
Management Responsibility
Design and Development/Design Controls
Control of Non-Conforming Products
Medical Device Reporting (MDR)
Production and Process Controls (P&PC)
Monitoring and Measurement
Sterilization Process Controls
Purchasing Controls
Documentation and Records
Customer Requirements
Technical Files
Risk Management
Completion Page
5 Steps to Prepare for ISO 13485:2016 Certification
Now, what must organizations keep in mind and consider when getting ready to obtain a certification in ISO 13485? Here are some steps they can take:
Obtain a copy and gain an understanding of the ISO 13485:2016 standard .
Identify areas for improvement in the current QMS by conducting a gap analysis or a readiness audit to ensure compliance with the ISO 13485:2016 regulatory requirements.
Perform quality monitoring audits and maintain a record of the results.
Define your organization’s competencies and determine training requirements for ISO 13485:2016 certification based on the internal audit results.
Ensure competence needs are met and that all parties involved are kept in the loop.
It’s also crucial to note that these steps aim to guide your organization on what to prioritize doing toward an ISO 13485 certification. However, you may add more processes or necessary audits to complement your efforts in maintaining a working and safe QMS.
How to Use a Checklist for ISO 13485 Compliance
Here’s a step-by-step guide on how to use an ISO 13485 audit checklist:
Familiarize yourself with the checklist to understand the questions or criteria it includes. Ensure that you have a clear understanding of the requirements of ISO 13485 to effectively use it, too.
Assess your current processes and activities against the checklist. Compare your practices with the requirements outlined in the checklist and identify areas where your organization may have gaps or areas for improvement.
Document evidence to demonstrate compliance with each checklist item. This may include records, procedures, work instructions, training records, or other relevant evidence.
Conduct a self-assessment of your organization’s QMS and determine whether your organization meets the requirements or if there are areas that need improvement.
Identify specific corrective actions to address issues such as non-compliance or areas requiring improvement and develop an action plan for implementing the necessary changes and improvements.
Execute the corrective actions according to the action plan to ensure that the identified improvements are implemented effectively and within the designated timelines.
Validate the effectiveness of the changes by conducting internal audits or reviews to ensure that the improvements have addressed the non-compliances and have resulted in a more compliant QMS.
Continuously monitor and review your QMS to ensure ongoing compliance with ISO 13485.
Regularly review and update the checklist to align with any revisions in the ISO 13485 standard or changes in your organization’s processes.
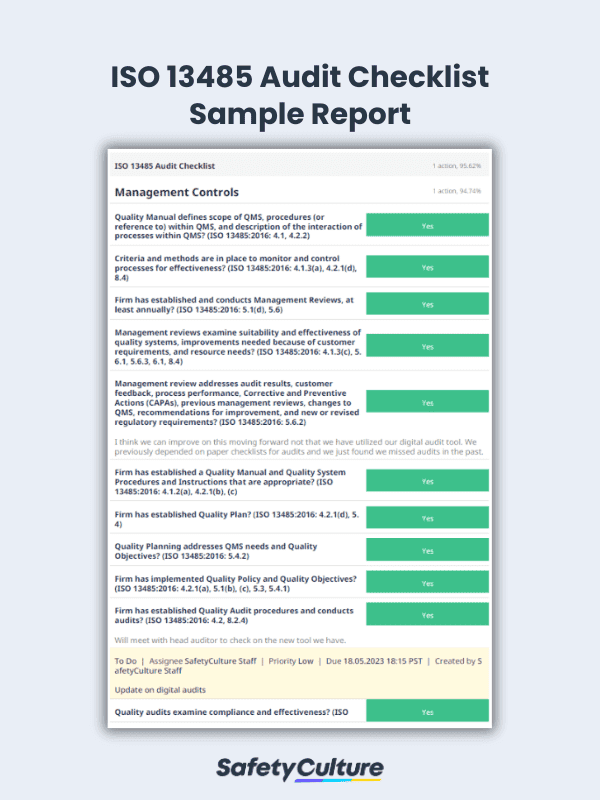

Thermosash was able to achieve ISO certification in six months using the SafetyCulture platform by centralizing workflows, boosting visibility, enhancing accountability, and streamlining quality control through QA checks and automatic corrective action alerts.