How to Identify Non-Conformance at Work
Learn about nonconformity, examples, ways to prevent, and procedure.

Published 3 Apr 2025
Article by
7 min read
What is Non-Conformance?
Non-conformance or nonconformity is the failure to meet specified requirements. Nonconformity can occur on both the process and the product. Consequently, nonconforming procedures such as not utilizing the management system correctly or not following the standard operating procedures can lead to nonconforming products.
What are Examples of Nonconformity?
Non-conformance is typically categorized into two different types—minor non-conformance and major-non-conformance. What is the difference between these two?
Minor Non-conformance
This is considered to be minor failures that would unlikely lead to major consequences. Most minor non-conformances are isolated cases and are easier to solve. Examples of minor nonconformity for processes are instances such as:
unauthorized change in documentation
missegregation of non-conforming products
improper machine calibration
incorrect sequence of minor processes
non-critical defects of product packaging
Major Non-conformance
This is a significant failure to meet quality requirements and major standards such as ISO 9001. Major non-conformance mistakes adversely affect production processes and products which can be costly to the company. Examples of major nonconformity are incidents such as:
frequent unauthorized changes
shipping out of untested products
missing critical documentation
working in unsafe environmental conditions
personnel who failed to take corrective action on a root cause of an issue
When any of these incidents occur, a Non-Conformance Report (NCR) is necessary to document and take action. A Non-Conformance Report is a detailed document that addresses the failure to meet quality standards. In an NCR report, it’s important to detail how the incident happened and how to prevent it from happening again in the future. Here’s an example of an ISO 9001 checklist that can help ensure the facility’s processes and Quality Management System (QMS) are in compliance with the ISO standard.
ISO 9001:2015 Audit Checklist | SafetyCulture
How do you Handle Nonconformities?
The simple answer is to fix the mistake and ensure that processes and products are in compliance with the ISO 9001:2015 standard. The FDA recommends corrective and preventive action (CAPA) for severe non-conformance that negatively affects design and manufacturing. It is not required to document the non-conformance procedure, however, nonconformities and the corrective action that was conducted have to be recorded along with other related information according to the ISO 9001 standard.
Improve your GRC management
Simplify risk management and compliance with our centralized platform, designed to integrate and automate processes for optimal governance.
Tips to Prevent Non-Conformance
Lessen the possibility of nonconformity with the following tips:
Adhere to the standard operating procedure – Employees are expected to follow the production processes set by the company since most of these procedures incorporate controlled variables that are proven effective when producing quality products. This keeps operations running smoothly. SOPs should be written and made available to every staff member in the workplace. Employers should also remind employees to report any changes to these procedures even if it’s just part of the documentations since unauthorized change in documents can lead to nonconformity.
Proper training – Employers have to train their employees with procedures that are in compliance with ISO 9001. This is necessary to prevent mistakes that could lead to non-conformance since minor details such as incorrect calibration can affect production.
Test the products – A concrete method to ensure ISO 9001 compliance is to regularly test the products. This is a best practice for quality assurance and control. Product testing will prevent nonconforming products from being delivered and sold to customers.
Consistently use an ISO 9001 Audit Checklist – An audit checklist or report form helps employees remember and complete every instruction, process, and necessary steps.
Scale Your Enterprise Operations with Customizable Solutions
✓ Scale ✓ Data ✓ Security ✓ Integration ✓ Teams
The Building Blocks of Workplace Conformance: Training with Policies and Procedures
Policies and procedures set out the rules and guidelines that your workers should follow to secure workplace efficiency, as well as guarantee everyone’s health and safety. The best way to engrain these guidelines into your workers is by putting together robust training on ensuring conformance with set standards, policies, and procedures.
By reinforcing your policies and procedures through training, workers will be better equipped with the knowledge and skills needed to identify potential hazards. With this training, they’ll be able to take the appropriate measures to prevent accidents and process issues from ever occurring. This can lead to a mindset shift, where your policies and procedures become a natural part of their work habits, creating a safer work environment for everyone.
Creating effective training is quick and easy – as long as you have the right tool in hand. For that, try SafetyCulture (formerly iAuditor)’s Training. Create and deploy training courses and programs to your employees to ensure they follow best practices and help achieve conformance in the workplace.
You can also make training courses accessible to your team using their preferred devices. With Training’s offline access, they can brush up on SOPs even without an internet connection.
Non-Conformance Procedure
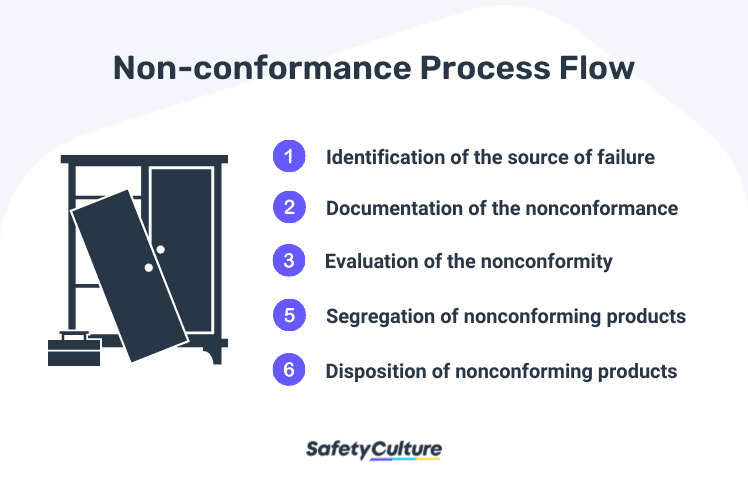
The non-conformance process flow includes 5 steps:
Identification of the source or root cause of the failure.
Documentation of the non-conformance such as the material, problem, disposition, and signature of the person responsible. There should also be a documented standard operating procedure and work instructions .
Evaluation of the nonconformity and determine if an investigation is required. An investigation is not needed when a similar situation has already been investigated; however due to the recurrence, a corrective and preventive action (CAPA) procedure may be required (CFR 829.100).
Segregation of nonconforming products.
Disposition of nonconforming products (CFR 820.90(b)).
Personnel should be ready to take corrective action after completing these steps.
Non-Conformance Process Flow | SafetyCulture
Non-Conformance Report Example
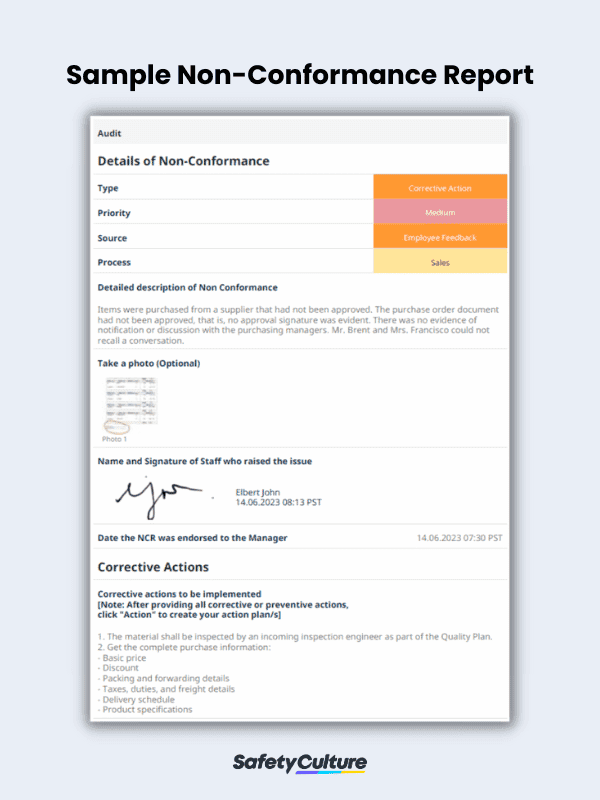
Utilize a non-conformance report form to document necessary details regarding nonconforming processes and products. This report helps quality managers gather data that is required for ISO 9001 such as the description of the non-conformance situation, corrective action, and signature of the personnel. In the event that the non conformity case is difficult to describe, staff can take photos and upload them to the form. This enables accurate recollection of the non-conformance issue.
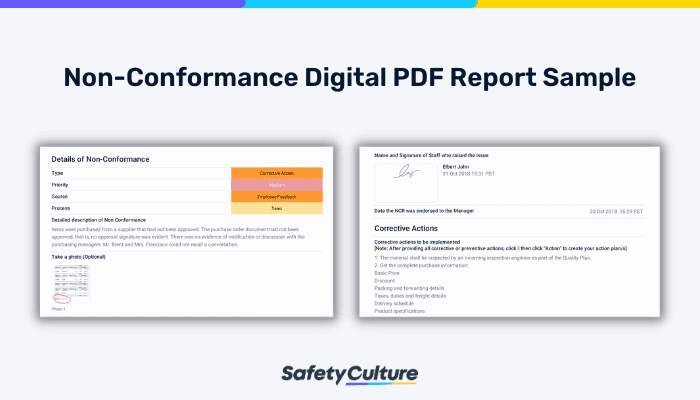
Non-Conformance Digital PDF Report Sample | SafetyCulture
Create your own Non-Conformance Report Template
Build from scratch or choose from our collection of free, ready-to-download, and customizable templates.
Report Non-Conformance with SafetyCulture
It is important for a company to keep documents organized especially for processes that majorly impact day-to-day operations. Nonconformity cases need to be documented to meet standards such as ISO 9001:2015. SafetyCulture is the leading quality management tool used for conducting inspections and filing reports. Other key QMS features include:
Customizable non-conformance report forms
Scheduling for product testing
Adding detailed instructions for the personnel
Ability to upload media files such as images
Determine the corrective actions that need to be implemented to fix the nonconforming issue
Name and signature of the person filling out the report
Data Analytics to view overall quality management performance, asset management insights, and corrective actions that needs to be fulfilled and also observe for recurring non-conformance issues
Featured Templates
Nonconformity-related Templates
Here are two examples of nonconformity-related templates for minor and major non-conformance situations:
Minor non-conformance – ISO 9001:2015 Corrective Action Report
Major non-conformance – CAPA Report
Article by
SafetyCulture Content Team
SafetyCulture Content Contributor, SafetyCulture
View author profileIn this article
- What is Non-Conformance?
- What are Examples of Nonconformity?
- How do you Handle Nonconformities?
- Tips to Prevent Non-Conformance
- The Building Blocks of Workplace Conformance: Training with Policies and Procedures
- Non-Conformance Procedure
- Non-Conformance Report Example
- Report Non-Conformance with SafetyCulture
- Featured Templates
Related articles
Compliance
Construction Site Compliance

Build a Solid Foundation with Caisson Construction
Find out how caisson construction can provide a stable foundation for your next project in a quick, cost-effective, and reliable manner.
Compliance
ISO

A Brief Guide to ISO 22000 Training
Explore the factors, requirements, and best practices for delivering impactful ISO 22000 training and implementing systems effectively.
ISO
Compliance

Comprehensive Guide to ISO 45001 Training for Work Safety
Explore the benefits of delivering ISO 45001 training across multiple industries and choose what works best for your team.