ISO 17664: Your Guide to Proper Medical Device Sterilization
This guide covers ISO 17664, its role in manufacturing, why it’s split into two parts, and how to properly sterilize medical devices.

Published 9 May 2025
Article by
6 min read
What is ISO 17664?
ISO 17664:2021 is an internationally recognized standard that outlines the information manufacturers must provide on how to properly clean, disinfect, and sterilize medical devices. This includes everything from high-risk devices like scalpels, endoscopes, and injection tools to low-risk items like wheelchairs.
The standard emphasizes the importance of giving clear, detailed instructions for safe processing by using the right cleaning methods and updated technologies. By following these guidelines, manufacturers help ensure devices are safe before they ever reach hospitals or clinics.
Why is ISO 17664 Split Into ISO 17664-1 and ISO 17664-2?
The ISO standard for cleaning, disinfecting, and sterilizing medical devices was split into two parts to clearly distinguish the requirements for critical versus non-critical devices. ISO 17664-1 covers critical and semi-critical medical devices, while ISO 17664-2 focuses on non-critical medical devices. This is because the more critical the device, the higher the risk of infection if not properly processed.
Here are examples of critical, semi-critical, and non-critical medical devices based on the standard:
Critical medical devices (high risk of infection):
Needles and Syringes
Implants
Dialysis machines
Semi-critical medical devices (moderate risk):
Endoscopes
Respiratory therapy equipment
Laryngoscopes
Dental mirrors
Non-critical medical devices (low risk):
Stethoscopes
Thermometers
Wheelchairs
Hospital beds
This split helps manufacturers give clear reprocessing instructions and helps users follow the right procedures based on the device’s risk level. However, keep in mind that each device has its own specific cleaning requirements.
The Role of ISO 17664 in Safe Medical Device Production
Sterilizing medical devices is crucial, as these tools are often injected or inserted into the body. Whether single-use or reusable, they must be thoroughly cleaned to prevent infection or complications, in line with ISO 13485 and manufacturing safety standards. Today, there is an increased focus on validating each step to ensure medical devices are properly cleaned, disinfected, and sterilized—supported by ISO 17025 to ensure the accuracy and reliability of testing and calibration procedures.
Manufacturers need to keep the common standards for manufacturing in mind when building medical devices. Under these, medical devices:
Must be easy to clean: The device should be simple to disassemble and clean after each use, all while keeping the physician’s workflow in mind.
Must be tested and documented: Everything from temperature tolerance to detergent compatibility needs to be thoroughly tested and documented before sale to ensure it holds up to real-world cleaning procedures.
Must have clear instructions: The standard requires detailed Instructions for Use (IFU), covering every stage of reprocessing—from point-of-use to storage .
Must align with government regulations: Devices also need to meet regulatory requirements from bodies like the Food and Drug Administration (FDA) in order to be marketed legally.
Improve your EHS Management
Cultivate a safe working environment and streamline compliance with our EHS solutions.
How to Conduct a Sterilization Process
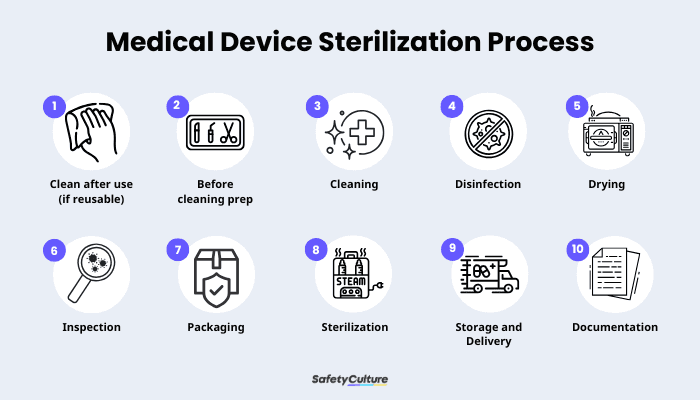
Medical Devices Sterilization Process
ISO 17664 requires manufacturers to provide a detailed instruction for the sterilization process of each medical device. While the process may vary depending on the device, here are some general guidelines set by manufacturers standardized across different devices:
Initial Treatment at Point of Use:
Remove any visible soil or blood immediately after use to prevent drying. Having other substances on a medical device can make it harder to clean later on.
Preparation Before Cleaning:
Disassemble devices as necessary and prepare any cleaning tools or sterilization methods according to the manufacturer’s instructions. Note each device will be different.
Perform Cleaning :
Clean the device thoroughly to remove all organic and inorganic material. Follow the sterilization instructions carefully, including specifications for water quality and the type of detergent to use.
Disinfection (if required):
Apply disinfection procedures to reduce the microbial load before sterilization. This is especially necessary for devices that will be handled further.
Drying:
Ensure devices are completely dry to prevent microbial growth and to eliminate the chance of recontamination. It’s essential to have at least one verified drying method specified.
Inspection and Maintenance :
Inspect the device for cleanliness, damage, or loose parts. After, perform any necessary maintenance as per manufacturer’s guidelines, ideally with the help of a digital checklist specific for the device.
Package the Device:
Package devices in materials compatible with the chosen sterilization method. This ensures they remain sterile throughout their shelf life or until the point of use.
Sterilization:
Use a validated sterilization method (steam, ethylene oxide, or radiation) as per each device’s manufacturer’s guidelines. The process must achieve a Sterility Assurance Level (SAL), typically defined as a one in a million chance that any viable microorganism remains after sterilization.
Store sterilized devices in a way that maintains sterility until use, whether in storage or during transport. The use of air quality sensors with real-time monitoring can be helpful for this.
Documentation and Validation:
Manufacturers must validate, document, and record each step of the process, and provide clear instructions and inspection criteria. Digital solutions like SafetyCulture can help streamline documentation and ensure consistency, as well as record and analyze data from medical devices in use and monitoring sensors.
Explore SafetyCulture Monitoring Solution
Utilize advanced sensor technology to monitor assets, automate vital alerts, implement actions, and report urgent issues.
Role of Technology in Ensuring Clean Medical Devices
While ISO 17664 is for manufacturers, consumers such as other businesses and those in healthcare should also be mindful of how they use and clean their medical devices. As medical devices become more advanced, the tools for cleaning them are evolving too. Sterilization processes are now more streamlined and efficient. For example,ultrasonic cleaners use high-frequency sound waves to reach tight or complex areas that are difficult to clean manually. On top of that, more manufacturers are developing eco-friendly cleaning products which deliver the same cleaning power while minimizing environmental impact.
Technology is also helping reduce mundane tasks and catch things that might otherwise be overlooked—like device maintenance,staff training,audits,fleet deliveries, and more. Advanced sensors can automatically set and monitor the right storage temperatures to help maintain a device’s sterility. Digital inspections can be completed and shared instantly via QR codes, cutting down on paperwork and the risk of lost documents. Altogether, these tools help ensure that safety protocols are followed both during manufacturing and at the point of use.
Ensure Proper Sanitization of Medical Devices with SafetyCulture
Why Use SafetyCulture?
SafetyCulture is a mobile-first operations platform adopted across industries such as manufacturing, mining, construction, retail, and hospitality. It’s designed to equip leaders and working teams with the knowledge and tools to do their best work—to the safest and highest standard.
Efficiently manage and streamline health and safety processes across the organization, including incident management, safety audits and inspections, risk assessment, waste management, and more, using a comprehensive EHS software solution.
✓ Save time and reduce costs ✓ Stay on top of risks and incidents ✓ Boost productivity and efficiency ✓ Enhance communication and collaboration ✓ Discover improvement opportunities ✓ Make data-driven business decisions
Related articles
Compliance
Construction Site Compliance

Build a Solid Foundation with Caisson Construction
Find out how caisson construction can provide a stable foundation for your next project in a quick, cost-effective, and reliable manner.
Compliance
ISO

A Brief Guide to ISO 22000 Training
Explore the factors, requirements, and best practices for delivering impactful ISO 22000 training and implementing systems effectively.
ISO
Compliance

Comprehensive Guide to ISO 45001 Training for Work Safety
Explore the benefits of delivering ISO 45001 training across multiple industries and choose what works best for your team.