What is GMP?
GMP stands for Good Manufacturing Practices, a system that ensures that manufactured products—such as food, cosmetics, and pharmaceutical goods—are consistently produced and controlled according to set quality standards. Implementing GMP can help cut losses and waste, and avoid recalls, fines, and jail time. Overall, it protects both the company and the consumer from negative food safety events.
GMPs examine and cover every aspect of the manufacturing process to guard against any risks that can be catastrophic for products, such as cross-contamination, adulteration, and mislabeling. Some areas that can influence the safety and quality of products that GMP guidelines and regulations address are the following:
- Quality management
- Sanitation and hygiene
- Building and facilities
- Equipment
- Raw materials
- Personnel
- Validation and qualification
- Complaints
- Documentation and recordkeeping
- Inspections & quality audits
What is the difference between GMP and cGMP?
Good Manufacturing Practices (GMP) and current Good Manufacturing Practices (cGMP) are, in most cases, interchangeable. GMP meaning the basic regulation promulgated by the US Food and Drug Administration (FDA) under the authority of the Federal Food, Drug, and Cosmetic Act to ensure that manufacturers are taking proactive steps to guarantee their products are safe and effective. cGMP, on the other hand, was implemented by the FDA to ensure continuous improvement in the approach of manufacturers to product quality. It implies a constant commitment to the highest available quality standards through the use of up-to-date systems and technologies.
What are the 5 Main Components of Good Manufacturing Practice?
It is paramount to the manufacturing industry to regulate GMP in the workplace to ensure consistent quality and safety of products. The five main components of GMP, commonly referred to as the 5P’s, help organizations comply with strict standards throughout the entire production process.
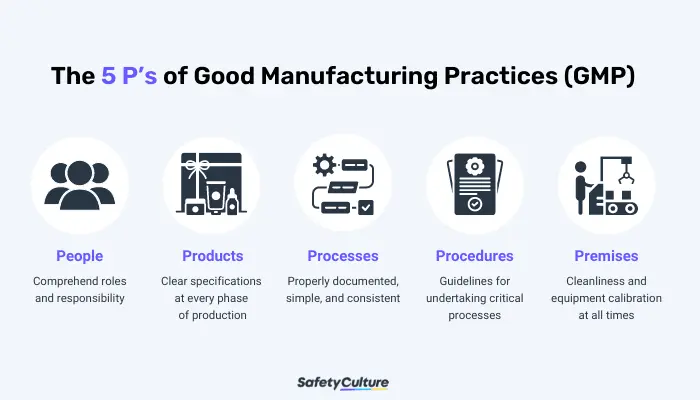
- People – All employees are expected to strictly adhere to manufacturing processes and regulations. A current GMP training must be undertaken by all employees to fully understand their roles and responsibilities. Assessing their performance helps boost their productivity, efficiency, and competency.
- Products – All products must undergo constant testing, comparison, and quality assurance before distributing to consumers. Manufacturers should ensure that primary materials including raw products and other components have clear specifications at every phase of production. The standard method must be observed for packing, testing, and allocating sample products.
- Processes – Processes should be properly documented, clear, consistent, and distributed to all employees. Regular evaluation should be conducted to ensure all employees are complying with the current processes and are meeting the required standards of the organization.
- Procedures – A procedure is a set of guidelines for undertaking a critical process or part of a process to achieve a consistent result. It must be laid out to all employees and followed consistently. Any deviation from the standard procedure should be reported immediately and investigated.
- Premises – Premises should promote cleanliness at all times to avoid cross-contamination, accidents, or even fatalities. All equipment should be placed or stored properly and calibrated regularly to ensure they are fit for the purpose of producing consistent results to prevent the risk of equipment failure.
What are the 10 Principles of GMP?
- Create Standard Operating Procedures (SOPs)
- Enforce / Implement SOPs and work instructions
- Document procedures and processes
- Validate the effectiveness of SOPs
- Design and use working systems
- Maintain systems, facilities, and equipment
- Develop job competence of workers
- Prevent contamination through cleanliness
- Prioritize quality and integrate into workflow
- Conduct GMP audits regularly
Further information can be found at this PDF Guide to GMP: Good Manufacturing Practices.
GMP Regulations
GMP regulations are mandated by manufacturers’ respective national governments to regulate the production, verification, and validation of manufactured products and ensure that they are effective and safe for market distribution.
For example, in the United States, GMP is enforced by the US FDA through Current Good Manufacturing Practices (CGMP) which cover a broader range of industries such as cosmetics, food, medical devices, and prescription drugs. The FDA conducts facility inspections to assess if a manufacturing company complies with CGMP regulations. If any serious violations are found during the inspection, FDA recalls all products, which is problematic for manufacturers in terms of both profit and business operations.
The quality of manufactured products is highly regulated as it can pose negative health risks to consumers and even the environment. Poor hygiene, temperature-control, cross-contamination, and adulteration in any step of the manufacturing process are some examples of how a manufactured product that doesn’t follow GMP regulations can bring fatal consequences to consumers. See GMP regulation and preamble sources by country here.
GMP Standards
GMP standards are developed to enhance the safety of manufactured products, especially pharmaceutical goods, and to ensure consumers get the highest quality possible. Adherence to GMP standards not only positively impacts the reputation of manufacturing companies but also reduces batch recalls and negative reports from consumers. Below are 4 measures you can follow to uphold GMP standards:
Quality team
Have a team of skilled workers that will focus on improving current manufacturing procedures and complying with GMP. Members will perform quality assessments on operations to identify problems and develop appropriate corrective measures. Part of the team’s responsibility will also be performing scheduled monitoring of instruments, equipment, processes, and staff skills.
Validation
Validation is the documented act of demonstrating instruments, processes, and activities that are regularly used or done. This is done to check if they function according to expectations. GMP can involve a number of things to be validated, but it’s good to focus on the following processes:
- Process validation
- Cleaning and sanitation validation
- Computer system validation
- Analytical method validation
Surprise Audits
A surprise audit every now and then can help gain a more accurate insight into what goes on in the facility. Identify real root causes of non-compliance and take action before it progresses into a larger issue. Read more about best practices in doing GMP audits.
Compliance Training
Providing compliance training to staff is the best way to ensure compliance with GMP standards. Help staff gain a better understanding of GMP and continually improve operations or systems in place to ensure standards are GMP-compliant. All employees should receive training on recordkeeping, sanitation, proper equipment handling, and labeling, and SOPs to minimize errors and maintain compliance.
Create your own GMP Audit Checklist
How to Comply with Guidelines
GMP guidelines and regulations address different issues that can influence the safety and quality of a product. Meeting GMP or cGMP standards helps the organization comply with legislative orders, increase the quality of their products, improve customer satisfaction, increase sales, and earn a profitable return on investment.
A GMP audit helps improve the overall performance of different systems including the following:
- Building and facilities
- Materials management
- Quality control systems
- Manufacturing
- Packaging and identification labeling
- Quality management systems
- Personnel and GMP training
- Purchasing
- Customer service
Ensure Compliance with Manufacturing Regulations
GMP Training
We’ve curated a list of GMP training courses that will guide your employees in following GMP standards so they can be proactive in minimizing risks in all aspects of manufacturing products such as food, cosmetics, and pharmaceutical goods. These courses cover topics such as good manufacturing practices, quality control, risk management, and many more.
Customer Story
See how a trusted food delivery business in Australia, Marley Spoon, immediately takes action based on real-time data using SafetyCulture temperature sensors:



