What is a Corrective Action Plan?
A corrective action plan is a documentation used in quality management that outlines a set of steps for addressing issues and gaps in business operations and processes that could negatively impact the business . It describes the approach for resolving an issue that interferes with reaching company goals. The corrective action plan should be S.M.A.R.T. (Specific, Measurable, Attainable, Relevant, Timebound) and includes timeframes, costs, and signatories.
What is a Weak Corrective Action Plan?
A strong corrective action plan doesn’t depend on uncertain variables which could make the corrective action plan weak. According to the Department of Veterans Affairs National Center for Patient Safety’s root cause analysis, a weak corrective action plan depends on current workplace processes and employee training.
Examples of enforcing employee training and policies are:
- Staff conducting double checks
- Staff retraining
- Creating new work procedures
How to Create a Plan
Creating a corrective action plan can seem to be a daunting task at first, and the persons responsible could benefit from ideas about where to begin. Generally, there are 3 different ways you and your team can try to start developing a corrective action plan:
Practice How to Identify
Another approach that can help you continue developing a corrective action plan is to go through corrective action identification activities. Training your teams to be able to correctly identify the need for a corrective action is a worthwhile endeavor that sets them up for success. Collaborate with your team in determining the root cause and the elements that need to be considered when developing the corrective action plan and implementing corrective action management, among other things.
Discuss Guide Questions
Following the 4 Ws and 2Hs format can be helpful in crafting a more comprehensive corrective action plan. Consider asking these sample questions to facilitate a planning session with your team:
- What should be done to correct the issue?
- Who should be responsible for implementing these corrective actions?
- When and how often should the corrective actions be carried out?
- Where should the corrective action documentation take place?
- How can we make sure that these corrective actions prevent recurrence?
Use a Digital Template
One of the quickest ways to help you get started with your corrective action plan is by using a template. A corrective action plan template is a useful tool that enables teams to easily document a course of action for a non-conformity. Take note that utilizing a template can jumpstart the creation of a corrective action plan, and it still needs to be finalized and reviewed.
Corrective Action Plan Examples
While corrective actions come in many forms, they usually reflect in a Corrective and Preventive Action Report Form and a Corrective Action Request Form. Here are a couple of examples of the two most common types of corrective actions:
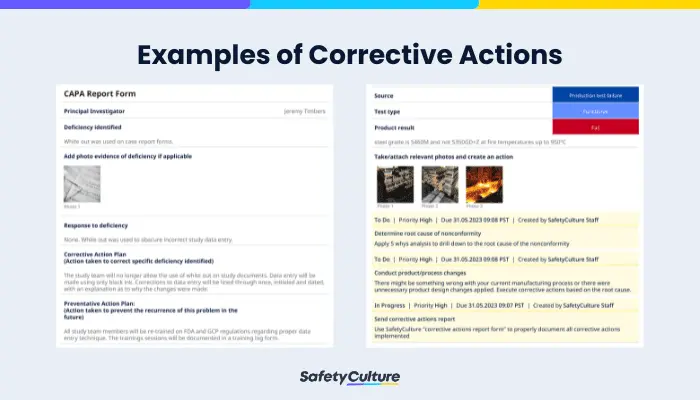
Corrective Action Examples: Corrective and Preventive Action Report, Corrective Action Request, and 8D Report
Here’s an example of corrective action (following the 4Ws and 2Hs).
| Briefly describe the problem |
| Reports of IV line errors have risen by 7% since the previous quarter. |
| What will be done? (Action steps, description) |
| In-house seminars will be held to retrain hospital personnel on safe and proper preparation of IV lines, pre-insertion guidelines, labelling of IV lines, placement, monitoring, as well as complete and proper documentation. |
| Why will it be done? (Justification, reason) |
| The seminars will serve to clarify SOPs regarding the preparation, utility, and monitoring of IV lines. It is also intended to reinforce our standards to improve safety and lower rates of preventable errors. |
| Where will it be done? (Location, area) |
| Providence Conference Room |
| When will it be done? (Time, dates, deadlines) |
| 7 seminars will be held for 30 minutes each to cover all shifts and personnel without adversely affecting staffing:
July 19 July 20 |
| Who will do it? (Who’s responsible?) |
| Doctor Barnes for July 19 (9am,11am,3pm) Doctor Sparrow for July 19 (5:30pm, 8:00pm) Doctor Maye for July 19 (11pm) and July 20 (3am) |
| How will it be done? (Method, process) |
| Doctor Barnes, Doctor Sparrow, and Doctor Maye will convene and create a 30-minute presentation on the proper proper preparation of IV lines, pre-insertion guidelines, labelling of IV lines, placement, and monitoring to be presented at intervals stated above. |
| How much? (What will it cost to do/make?) |
| No extra expenses. |
Here are a few other corrective action examples that specifically deals with workplace safety and business security:
- Providing equipment upgrades
- Implementing safety training
- Conducting regular and random safety audits
- Updating work processes
- Revisiting safety protocols and guidelines
How to Create a Report
Based on the given corrective action examples, here are some tips on how to create corrective action reports and the most basic information that should be captured by the reports:
- Determine and record the issue or non-conformance that prompted the need for corrective action.
- Taking pointers from creating a corrective action plan, capture the details of the corrective action to be taken to rectify the non-conformance.
- Submit the corrective action report to the intended recipients and keep a record for future reference and follow through.
Create Your Own Corrective Action Report
Eliminate manual tasks and streamline your operations.
Get started for FREEHow Is It Different from Preventive Action?
While both intend to address non-conformance, there are some differences between corrective and preventive action.
| Corrective Action | Preventive Action |
| Considered the “reactive” way of addressing non-conformance | “Proactive” way of addressing non-conformance |
| Corrects a non-conformance that already happened | Aims to keep a non-conformance from happening in the first place |
| Both corrective action and preventive action intend to avoid the re/occurrence of non-conformance | |
How Do They Work in ISO 9001?
Corrective actions are implemented to identify, resolve, and prevent issues of quality non-conformity. In prior ISO 9001 standards, Corrective and Preventive Actions (also known as CAPA) were separate action plans that each had their own requirements. Summarized in a CAPA Report, they outline the plans on how to fix the existing problem immediately and how to prevent it in the future.
However, the latest ISO 9001 standards (ISO 9001:2015 Clause 10.2) eliminated the need for predefined procedures for preventive actions. Instead, it emphasized the importance of having a risk-based approach throughout the process, rather than “preventive actions” being done and documented separately.
The 5-Step Process: How to Implement for ISO
In this simplified guide for quality managers, we present 5 steps on how to implement corrective actions that are compliant with ISO 9001:2015, with the help of a compliance software.
Step 1: Determine if the situation calls for it.
Not every issue warrants a corrective action. The purpose of issuing a corrective action plan is to identify and resolve problems that are systemic, something that endangers a company’s Quality Management System software.
Quality managers and their teams can choose an appropriate risk analysis technique to determine the severity of a non-conformity and decide if a corrective action is needed. The most frequently used approaches are FMEA and HAZOP. This is a good way to inculcate risk management right at the start of the process and help qualify the level of risk and impact an issue brings to the organization, product, and/or services.
Once a corrective action is deemed necessary, the quality team and process owners can collaborate and use an ISO 9001:2015 – compliant corrective action report template to document their plan’s progress.
Step 2: Conduct root-cause analysis (if applicable).
Root-cause analysis (RCA) is a methodical approach in analyzing a problem and identifying the root causes to solve counterproductive issues or events. It is based on the belief that issues are best solved by eliminating the root cause/s, as opposed to merely addressing the apparent symptom. RCA is best used for issues that cannot be resolved quickly, are repetitive, and systemic.
ISO 9001:2015 highly recommends engaging cross-functional team members and leaders throughout the planning of corrective actions. Quality teams can encourage this by using a compliance software to facilitate collaboration, even when they’re in different locations.
The 5 Whys and/or 8 Disciplines of Problem-Solving (8D) approaches are good root-cause analysis techniques that can help them work together on crafting a good problem statement, identifying the root cause/s, and brainstorming on appropriate solutions to address non-conformance.
Step 3: Work on the plan as a team.
Identifying the root causes and their effect can help formulate the most appropriate corrective action/s. In a corrective action plan, there are two key actions: a “correction” recommendation to immediately eliminate a detected non-conformity and the corrective action itself.
Apart from the details about the non-conformity and the recommended corrective action, specific details should also be provided, such as implementation timeline, key people and signatories, and costs. Once finalized and reviewed, the digital corrective action plan can be shared to key people to standardize information.
Step 4: Communicate and implement it.
Enhance leadership involvement and encourage them to communicate the change and the rationale behind it. Notify key people via the QMS software about their assigned tasks. Provide a communication channel where anyone can give regular feedback while the corrective action is being implemented. These all factor in and determine the success of a corrective action plan’s implementation.
Step 5: Conduct follow-ups to ensure effectiveness.
IS0 9001:2015 requires organizations to review the effectiveness of corrective actions and update risk levels and possible opportunities. After the implementation, the process owners and quality team should wait a suitable amount of time and conduct follow-up reviews.
Additional fields in the digital corrective action plan are available so quality managers can input comments during the review. To document results, they can use QMS software features, such as attaching photos and notes, to provide clearer evidence on the effects of the implemented corrective action.
To implement corrective action plans that are compliant with ISO 9001:2015, quality managers can use a collaborative compliance software like SafetyCulture (formerly iAuditor) to manage their implementation — from root-cause analysis to review of effectiveness of corrective action plans.
FAQs about Corrective Action
Here are a few other corrective action examples that specifically deals with workplace safety and business security:
- Providing equipment upgrades
- Implementing safety training
- Conducting regular and random safety audits
- Updating work processes
- Revisiting safety protocols and guidelines
The 3 elements of corrective action are as follows:
- Problem identification – this involves clearly defining the problem or non-conformity that needs to be addressed.
- Root cause analysis – this identifies the underlying cause or causes of the problem. It involves thorough investigation of factors that contributed to the occurrence of the problem.
- Corrective action plan – the plan outlines the specific steps to be taken to address the identified problem. It should also clearly state responsibilities, timelines, and necessary resources to resolve the root cause of the problem.
A corrective action should be issued when a problem or non-conformity has been identified and requires investigation and resolution. Some example situations when a corrective action is issued are:
- When a customer raises a complaint about the product or service
- When deficiencies, deviations, or areas of improvement are identified during internal audits and inspections
- When accidents, near misses, or safety hazards occur in the workplace
- When a process outcome consistently deviates from the desired or set targets
- When quality issues arise despite having quality control measures in place
- When performance metrics or goals are not being met
The responsibility for implementing corrective actions will typically fall on the individuals or teams that are directly involved in the process or system where the problem was identified.
Yes, documenting corrective actions is crucial for ensuring traceability, accountability, and future reference. It plays a vital role in maintaining a comprehensive record of identified problems, the implemented solutions, and their respective outcomes.




