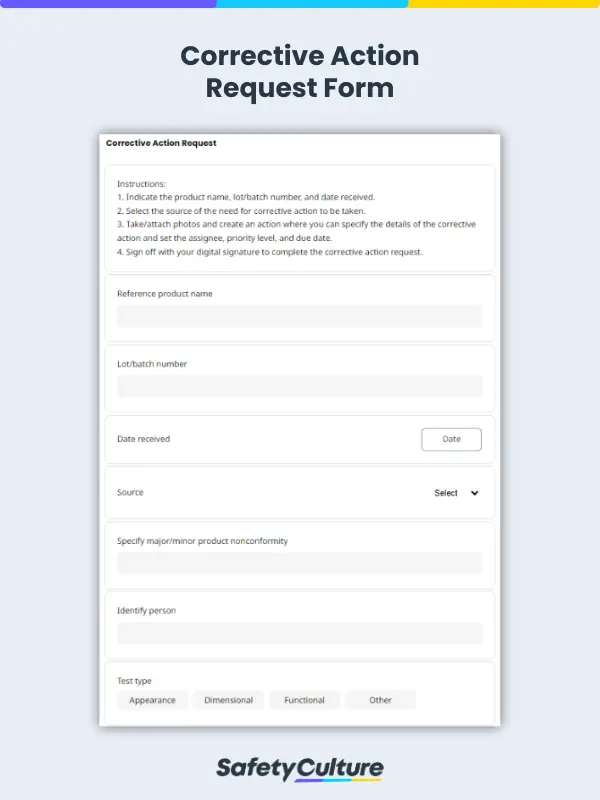
What is a Corrective Action Request?
A corrective action request is a formal notification sent to the supplier for rectifications to be done on a nonconforming item, process, or service. Suppliers respond to a corrective action request to report the root cause of a nonconformity and the applied corrective actions to prevent recurrence.
Monitoring and updating paper-based corrective action requests and corrective action reports can be time-consuming and burdensome for quality managers from both parties. Taking advantage of mobile-ready corrective action request forms can help easily track quality issues, mitigate risks of product recalls, and document corrective actions for ISO certification or compliance.
When to Issue Corrective Action Requests
Quality managers often overlook crucial situations that should trigger the issuance of corrective action requests because of administrative pitfalls such as filling out paper forms leading to the slow turnaround of reports and deferred rectifications to quality issues. Here are what quality managers should look out for prior to the issuance of corrective action requests:
Contractual Non-conformities
Quality inspectors usually detect a nonconforming item, process, or service during quality assurance inspections with the use of a manufacturing quality control checklist. Performing an incoming inspection helps easily determine if materials or components from a supplier meet purchase order specifications and other contractual requirements. Request for corrective actions to be taken when quality issues that can affect the fit, form, or function of products are found.
First Article Failures
Conduct a first article inspection in the manufacturing facility to verify the design characteristics of a product. If the production process has been proven to manufacture conforming products but the output from the first production run demonstrates nonconformity, corrective action requests can be sent to the supplier. Indicate that the root cause of the nonconformity should be identified and the corrective action report should be returned with proper documentation, especially for ISO certification or compliance.
Poor Performance
Issue a corrective action request for supplier deficiencies such as delayed deliveries of materials or components and prolonged closure times of quality issues. In creating a corrective action request, clearly specify the problem statement and its impact if left uncorrected. Recommend the completion of a factory acceptance test from the supplier to immediately resolve nonconformity and avoid costly production delays.