Process Validation
Learn about the stages, types, and examples of process validation, the guidance of FDA and GHTF for validating pharmaceutical manufacturing processes, and how to effectively establish documented evidence of process validation.

Published 11 Nov 2025
Article by
8 min read
What is Process Validation?
Process validation is a step-by-step procedure designed to ensure that a manufacturing process can consistently produce quality products. It is performed by a validation team led by the quality assurance head of manufacturers in the pharmaceutical industry. Generally, process validation is done before releasing a new product, when applying any change on an existing product, and for periodically verifying the process. Established at the onset, a protocol should specify how the validation process will be carried out, including the parameters to be monitored, the samples to be taken, and the results to be accepted.
Importance of Process Validation
Process validation is a cornerstone of quality assurance in manufacturing, particularly in industries like pharmaceuticals and biotechnology. It ensures that production processes consistently yield products that meet predefined quality standards and specifications. When a process validation is properly implemented, manufacturers are able to:
Ensure Product Quality – Validates consistent product quality.
Compliance – Ensures adherence to GMP and regulatory guidelines.
Enhance Product Safety – Identifies and mitigates safety risks.
Cost Efficient – Reduces waste and operational costs.
Continuous Improvement – Facilitate process optimization.
process validation infographic
Phases of Process Validation
Process Validation Guidance: FDA vs GHTF
The United States Food and Drug Administration ( USFDA ) guidance discusses general principles and practices that manufacturers can use to validate manufacturing processes. It covers different categories of drugs such as human, veterinary, and biological or biotechnology products.
A voluntary organization of regulatory agency officials called the Global Harmonization Task Force ( GHTF ) provided process validation guidelines for medical devices. It aimed to support manufacturers in understanding requirements concerning manufacturing, servicing, and installation processes for medical devices. Eventually, the International Medical Device Regulators Forum ( IMDRF ) replaced the GHTF, taking over its mission to standardize medical device regulations around the world.
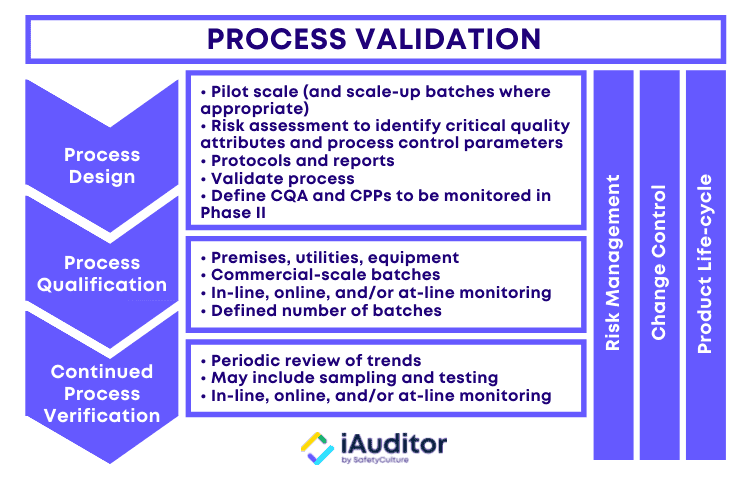
3 Stages of Process Validation
The USFDA emphasizes the collection and evaluation of data in their definition of process validation. It is crucial for the validation team not only to gather information about the activities throughout the lifecycle of the product and process, but also to analyze them for understanding the origins of variation and controlling it accordingly. The 3 stages of process validation are process design, process qualification, and continued process verification:
Stage 1: Process Design
During this stage, the manufacturing process is defined in a way that it can reproduce the delivery of a medicinal product that meets pre-determined specifications and quality attributes. To achieve this, the validation team should have a clear grasp of how the process actually works. Consider the following sources and methods to capture process information:
Product development activities
Functionality and limitations of production equipment
Predicted contributions to variability
Design of experiment (DOE) studies
Risk analysis tools
Experiments or demonstrations at laboratory or pilot scale
Computer-based or virtual simulations
This stage also involves process control, planning strategies to reduce input variation and/or adjust for it during manufacturing. Controls commonly consist of material analysis and equipment monitoring at significant processing points. In some cases, the use of process analytical technology ( PAT ) may be needed.
Stage 2: Process Qualification
The process qualification stage of process validation entails process design evaluation to determine if it is effective for quality production. First, the manufacturing facility should be designed according to the requirements of current good manufacturing practice ( CGMP ). Next,qualification of utilities and equipment should be conducted such as making sure that they are built and installed in compliance with design specifications. Finally,process performance qualification should be executed through a protocol and documented in a report:
Introduction, Objectives, and Scope
Production, Quality Assurance (QA), and Quality Control (QC) Responsibilities and Prerequisites (e.g. manufacturing records)
Process Validation Study Plan based on Quality Risk Management (QRM)
Validation Team (Research and Development, Engineering, Production, QA, and QC)
Product Details and Design Considerations
List of Materials, Vendors, and Master Formula
List of Equipment and Process Flow Chart
Brief Manufacturing Process and Assessment of Critical Process Parameters (CPP)
In-process Test and Specifications or Critical Quality Attributes (CQA)
Sampling, Testing Plan, and Acceptance Criteria and Limit
Yield Details (a minimum of three consecutive batches)
Analytical Method Validation
Finished Product Specification
Review of Out-of-Specification ( OOS ) Test Results, Deviation, and Change Control (CC)
Revalidation Criteria
Review of Follow-up Action (if any)
Summary and Conclusion
Stage 3: Continued Process Verification
After process design and process qualification, the third stage of process validation deals with setting systems to continually ensure that the validated process remains in such a state during routine production. Continued process verification often incorporates the use of statistical process control (SPC), the continuous monitoring and sampling of process parameters and quality attributes, and the scheduled maintenance of the facility, utilities, equipment, and related assets. It is essential for good documentation practices to be employed throughout the validation process.
Drive Excellence in Manufacturing Quality
Raise the bar for product quality and exceed customer expectations with standardized checks across all production sites.
4 Types of Process Validation
Process validation is often categorized according to the time it is performed in relation to the production schedule. Based on this description, there are 4 types of process validation: prospective validation, retrospective validation, concurrent validation, and revalidation.
Type 1: Prospective Validation
It is implemented when any product will be manufactured with a new formula or within a new facility. Also known as premarket validation, prospective validation is usually carried out before commencing routine production. It is also considered as the foundational type of validation because it is the starting point for any product that will be released under new conditions.
Type 2: Retrospective Validation
It is conducted only when the manufacturing process has not formally undergone a documented validation. Retrospective validation is normally fulfilled with the use of historical data and trends analysis to provide evidence that the process is at a state that it is intended to be in. In most cases, it is no longer an acceptable approach to process validation because any product should have already been validated before its commercial distribution.
Type 3: Concurrent Validation
It is done during regular pharmaceutical production to demonstrate that the process performs at the level that it should in the course of its actual execution. While concurrent validation is still an acceptable approach to process validation under certain circumstances (e.g. manufacturing medically necessary drugs in coordination with the USFDA to prevent a short supply), the agency continues to emphasize that it should only be used rarely.
Recently, an FDA inspection revealed that a US-based manufacturer produced adulterated drug products. To correct their violations, the pharmaceutical company responded with an intention to implement concurrent validation. However, the USFDA warned against it because they failed to show a clear understanding of variability sources in their manufacturing processes. Instead, the agency required them to comply with specific CGMP regulations, including adequately validating manufacturing processes.
Type 4: Revalidation
Revalidation is more widely used for medical devices than drug products. It is executed when prospective validation reaches a conclusion that the manufacturing process is unable to produce the product consistently. Moreover, a criteria for revalidation may be indicated in the original validation protocol. The revalidation process may not be as comprehensive as the initial validation, especially if the situation only calls for some aspects to be repeated.
Create your own Process Validation Report template
Build from scratch or choose from our collection of free, ready-to-download, and customizable templates.
Process Validation Examples
A validated process not only decreases the likelihood of batch failures, but it also increases the productivity of the manufacturing facility because of minimized rework and rejection. Examples of processes which should be validated include sterilization, aseptic filling, heat treating, plating, and plastic injection molding. In this pharmaceutical process validation example, a typical validation master plan for biotech processes contains:
Validation Master Plan Approval
Validation Master Plan Maintenance (Document Revisions and Revision History)
Definitions
Acronyms
Validation Master Plan Objective
Validation Master Plan Scope
Process Validation Strategy (Introduction, Support Qualification, Critical Process Parameters, Number of Runs to be Performed, and Non-conforming Results )
Process Validation Documentation (Protocols, Reports, Strategy Documents/ Assessments, and Record Retention)
Roles and Responsibilities (Technical Services, Operations, Engineering, Quality Control Laboratory, Quality Assurance, and External Services
Process Description
Process Validation Description (Document and Support Qualification for Hold and Mixing Strategies)
Maintenance of the Validated and Qualified State (Schedule, Preventative Maintenance and Calibration , Annual Product Review, Change Control, Periodic Review, and Continuous Process Verification)
Using templates for process validation can also streamline the data gathering process and facilitate a more efficient analysis of the validation results. To help you and your team get started, download and use the top 3 process validation templates for free:
Improve Manufacturing Processes with a Digital Solution
Why use SafetyCulture?
SafetyCulture is a mobile-first operations platform adopted across industries such as manufacturing, mining, construction, retail, and hospitality. It’s designed to equip leaders and working teams with the knowledge and tools to do their best work—to the safest and highest standard.
Streamline processes, eliminate bottlenecks, enhance resource utilization, and build an agile and scalable infrastructure with SafetyCulture. Strive for operational excellence to boost competitive advantage, foster sustainable growth, and deliver long-term value.
✓ Save time and reduce costs ✓ Stay on top of risks and incidents ✓ Boost productivity and efficiency ✓ Enhance communication and collaboration ✓ Discover improvement opportunities ✓ Make data-driven business decisions
Related articles
Operations
Business Processes

A Guide to How Operations Automation Streamlines Workflows
Learn what operations automation is, which workflows require streamlining, and how it reduces errors to improve performance.
Business Processes
Operations

Yokoten: The Key to Quality Improvement
Get to know the basics of the Yokoten principle and how it accelerates continuous improvement by sharing known solutions across teams.
Operations
Business Processes

Implementing Value Management for Better Business Outcomes
Explore value management, its principles, benefits, and helpful strategies to drive peak performance and cost-efficiency.