What is ISO 15189?
ISO 15189:2012 is an internationally recognized quality standard for clinical laboratories. It specifies requirements for quality management and technical procedures in medical laboratories. The standard is designed to ensure the high quality of laboratory testing and to promote confidence in the results generated by medical laboratories.
It is based on the quality management principles of ISO 9001 but with specific requirements for medical laboratories. ISO 15189 applies to all types of medical laboratories, including those that provide diagnostic, therapeutic, and public health services.
ISO 15189 covers all aspects of quality management in medical laboratories, from the initial planning and design stage to the final disposal of laboratory waste. It includes requirements for laboratory facilities, staff training, and the management of laboratory information.
Why is it Important?
The ISO 15189 standard is important for many reasons. First, it provides guidance on how to set up and operate a quality management system for medical laboratories. This is important because it helps ensure that laboratories are able to consistently produce accurate and reliable results.
In addition, the ISO 15189 standard helps to harmonize the quality of medical laboratory services around the world. This is essential because it allows patients to receive the same high level of care regardless of where they live.
Finally, the ISO 15189 standard helps ensure the safety of laboratory workers. This is vital because laboratory workers are often exposed to hazardous materials. By adhering to the ISO 15189 standard, laboratories can help to protect their workers from potential harm.
Five Additional Criteria of ISO 15189 for Medical Laboratories
According to the ANSI National Accreditation Board (ANAB), ISO 15189 includes five additional requirements for medical laboratories. These are:
- Applying ethical practices
- Gathering information on a set of procedures, sample volumes, and containers
- Giving assessments on the results of tests related to patient care and diagnosis
- Collaborating between the clinical staff and the laboratory that is responsible to clinicians that are referring patient samples for testing
- Providing guidance on the various types of samples and tests that may be required in laboratories
What are the Benefits of Having ISO 15189 Accreditation?
There are many advantages to having ISO 15189 accreditation. Below are some of the many benefits the standard offers to medical laboratories.
Risk Reduction
With ISO 15189, it will be easier to identify potential risks and allow medical laboratories to plan and implement preventive actions to mitigate risks.
Improved Quality Management
The improved quality management system (QMS) will allow medical laboratories to identify errors and enact corrective measures quickly and effectively.
Increased Efficiency
By implementing ISO 15189, medical laboratories can streamline their processes and procedures, leading to increased efficiency and productivity.
Improved Customer Satisfaction
An improved QMS and increased efficiency enable laboratories to deliver better quality services and provide more accurate results to their clients. This, in turn, will lead to improved customer satisfaction.
Increased Marketability
As an internationally recognized standard, ISO 15189 accreditation will make a medical laboratory more marketable and attractive to potential customers. After all, it serves as proof of their competence and commitment to providing quality services in line with global benchmarks.
Improve your GRC management
Why is it Essential For Laboratories to Get Certified?
So, why is it important for laboratories to be ISO 15189 certified? There are several reasons:
- Helps ensure that the laboratory is providing accurate and reliable results
- Builds confidence in the laboratory among patients, doctors, and other stakeholders
- Helps the laboratory to attract new business and win contracts
Overall, ISO 15189 certification is important for laboratories because it helps to ensure they are providing accurate and reliable test results to their clients. This, in turn, helps build confidence in the laboratory and provide quality service to its patients or customers.
How to Get ISO 15189 Certification
In order to be eligible for ISO 15189 certification, a laboratory must meet all of the requirements set forth by the standard. Here’s how you can get an ISO 15189 certification:
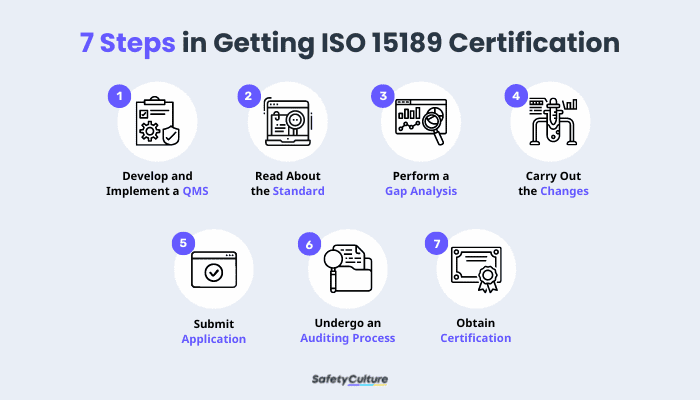
How to Get ISO 15189 Certification
Step 1: Develop and Implement a QMS
The laboratory must establish and carry out a QMS that meets the requirements of ISO 15189. The QMS must be tailored to the specific needs of the laboratory and must be documented in a form or template that is accessible to all staff members.
Step 2: Read More About the ISO Standard
Next, the laboratory must obtain a copy of the ISO 15189 standard and familiarize themselves with the requirements. Once the laboratory has a good understanding of the standard, it can begin the process of auditing its own operations to ensure successful accreditation.
Step 3: Perform Gap Analysis
Gap analysis is the process of comparing the current state of the laboratory against the requirements of the ISO 15189 standard. This will help the laboratory identify any areas that do not conform to or meet the requirements and will need to make changes.
Step 4: Implement Changes
Based on the results of the gap analysis, the laboratory will need to make changes to its QMS in order to meet the requirements of ISO 15189. These changes can be made through training, process improvement, or by implementing new policies and procedures.
Step 5: Submit Application
Once the laboratory has made all of the necessary changes to its QMS, it can submit its application for ISO 15189 certification. The application must be submitted to an accredited certification body, along with the required fees.
Step 6: Undergo an Auditing Process
Once the application has been submitted, the certification body will conduct an audit of the laboratory to assess its compliance with ISO 15189. The audit will consist of a series of site visits, interviews, and document reviews.
Step 7: Obtain Certification
If the laboratory is found to be in compliance with ISO 15189, it will be awarded the certificate. The certificate is valid for three years, after which the laboratory will need to undergo a re-certification audit.
FAQs for ISO 15189
ISO 15189 is voluntary unless required by law, regulation, or other contractual obligations. However, it’s still beneficial for laboratories to conform to this standard, as it aids them in enhancing the quality of medical services they provide.
Like any other ISO standard, it usually takes around three to six months to get an ISO 15189 certification. The time frame will also depend on the size and structure of the company.
- ISO 9001 is a quality management system standard that can be applied to any type of organization. It is not specific to laboratories.
- ISO 15189 is a quality management system standard specific to laboratories. It includes all of the requirements of ISO 9001 but also has additional requirements related to the specific nature of laboratory work.
- ISO 17025 is a laboratory accreditation standard. It includes all of the requirements of ISO 9001 and ISO 15189 but also has additional requirements related to the specific process of accreditation.
The standard is used by laboratory managers to be sure that the medical laboratory has a quality system that meets all legal requirements for operations. It is also used by accrediting and certifying bodies that assess a laboratory’s capability to perform the tests it claims it.


