A Short Guide to Current Good Manufacturing Practice (cGMP) Regulations
This article will discuss the purpose and importance of cGMPs and how it differs from other manufacturing processes.

Published 24 Feb 2025
Article by
6 min read
What is cGMP?
Current Good Manufacturing Practice (cGMP) regulations are defined by the US Food & Drug Administration (FDA) as guidelines set in place to ensure proper designing, monitoring, and controlling of manufacturing processes, facilities, and operations. Often used in the pharmaceutical industry, cGMP regulations are also present in other medical-related industries such as biotechnology and medical technology.
cGMP vs GMP
cGMP regulations are very similar to Good Manufacturing Practices ( GMP ) in that both seek to promote safety and quality. In some cases, they are also both used interchangeably as well, as they both follow the 5Ps:
People
Products
Premises
Procedures
Processes
However, there are small differences to note. GMP regulations have long been set and applied to all industries and tasks, and following them ensures that all parts of the process—from creating and keeping records to packaging—are done correctly, precisely, and safely. Meanwhile, cGMPs can be continuously changed depending on the circumstances and nature of work. This is because cGMPs are often created while considering the latest technology and innovations in mind, which are also subject to different changes, updates, and developments that happen over time.
The difference between them is also what warrants the small “c” for cGMP. The “c” is added to show that not only did the product created follow GMP guidelines, but the new and most current processes involved were carefully considered and evaluated as well.
Another difference in the meaning between cGMP and GMP is the cost involved. As cGMP takes into account new technologies, it can be more expensive to get certified for it as it would require more testing and evaluations. This is especially true for pharmaceutical products, as they would need to be tested more rigorously to ensure their safety.
Ensure Compliance with Manufacturing Regulations
Simplify internal audits, capture site observations, and address gaps in compliance to meet regulatory requirements.
Purpose of Having cGMP
Having cGMP guidelines to follow is just as important as having the standard GMPs in place. While GMPs and other testing procedures can be followed dedicatedly, the current trends and technologies must still be considered, especially in medicine-related fields.
Products in the pharmaceutical industry often require many rounds of testing, but testing is not always enough. Some things may have issues that could have been spotted earlier during the manufacturing phase or between steps. This is where cGMP comes into play. Not only does cGMP consider the technologies used in both production and testing, but cGMP regulations are also set in place to consider the working conditions of employees as well.
cGMP regulations call for employees to know all production processes and quality standards, as well as how to operate the machines they deal with daily. The different machines and forms of technology used and the people involved in their operation are included in total quality management so that companies and cGMP regulating bodies will have an idea if the products created are safe for consumption, even before testing is done. This way, quality is assured and improved upon every step of the way, and not just in the end.
cGMP and GMP vs GLP
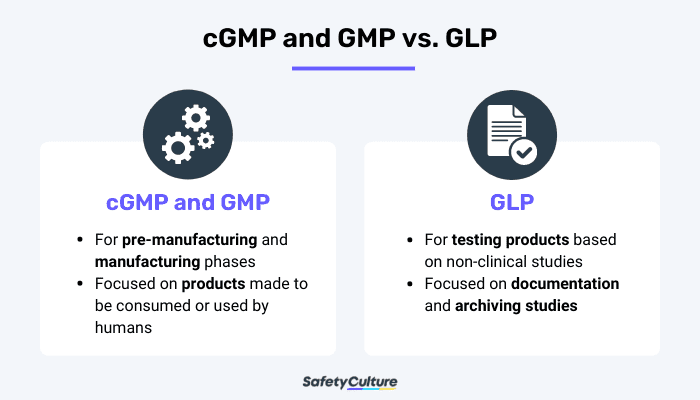
cGMP and GMP vs. GLP
cGMP and GMP regulations are sometimes confused with Good Laboratory Practice (GLP) due to both being necessary for the pharmaceutical industry. Their differences, however, are important to distinguish.
cGMP and GMP regulations are catered to the pre-manufacturing and manufacturing phases of a product. Although there are also regulations for testing, cGMPs and GMPs are more focused on the processes. On the other hand, GLP regulations are mainly for the testing of completed products. In the pharmaceutical industry, both are important to follow before releasing a product to the public market.
Another key difference is where they are used. cGMPs and GMPs can be applied to other fields besides medicine, as long as the products to be produced will be used by humans. GLP, meanwhile, focuses on the general testing of drugs based on non-clinical laboratory studies. Other things GLP focuses on are the documentation and archiving of studies and theories carried out in different laboratory settings.
Keeping Up with cGMP Regulations
Currently, if one wants a formal cGMP certification, they need to talk to recognized third-party accreditors. The FDA themselves do not issue certificates for both cGMP and GMP.
Create Your Own cGMP Audit Checklist
Regardless, the FDA calls for everyone to follow cGMP guidelines. Different products’ and industries’ respective cGMP regulations can be found on the FDA’s Guidance Documents page for everyone’s reference.
Products that do not follow cGMP regulations are not necessarily considered unsafe, but instead, can be called “adulterated” under the law. As an example, rather than telling people to stop consuming adulterated products, the FDA instead advises patients who are going through their drug therapy to not be interrupted, as doing so could cause them harm. In some cases, the FDA may call on the companies to recall their products, or sometimes, even stop distributing them entirely.
A good way to keep up with cGMP regulations is by using checklists. Digital checklists, specifically, can be a big help in keeping track of compliance with the FDA’s standards from any device and location. These checklists can also be used to note what documents to submit for accreditation.
Digital checklists can also help with auditing the current manufacturing processes in place, with or without applying for certification. Having checklists for internal audits can be a big help in improving existing processes, identifying issues early, and creating contingency plans.
SafetyCulture for Quality Assurance
For keeping up with cGMP regulations and cGMP requirements, as well as quality assurance, consider using a digital checklist app. SafetyCulture is an inspection application that you can use to create and use checklists. You also have the option to use pre-made templates from the Library and edit them as you please. You can also use your your existing Word, Excel, and PDF checklists on the app.
With the digital checklist on SafetyCulture, you can:
Assign scores and mark things as “failed” in case they don’t fit cGMP standards
Store created templates and finished inspections in one place
Inform others of problems, deviations, or other instances of non-compliance by raising Issues , assigning Actions , and providing a Heads Up to others
Schedule inspections
Generate analytical reports based on past inspections to help you better address any issues
Export inspection reports as Word or PDF files
SafetyCulture is also more than a checklist tool. With this application, you can create and conduct training sessions in-app as you also manage your inspections. At the same time, you can also manage your equipment and assets all in one place.
Featured Templates
Pharmaceutical GMP Audit Checklist
This checklist aims to help drug manufacturers with auditing their manufacturing facility and processes. Additionally, this checklist can be used to help measure compliance with GMP guidelines concerning the management, their employees, their equipment, and their security as well.
Related articles
Operations
Human Resources

The Role of HR in Workplace Health and Safety Management
Learn what an HR health and safety program covers, its key responsibilities, and the best practices for creating safer working environments.
Operations
Performance Evaluation

The RICE Framework: A Strategic Approach to Prioritization
Learn how the RICE framework and prioritization method helps put focus on high-ROI tasks to streamline workflows.
Operations
Business Processes

Implementing Value Management for Better Business Outcomes
Explore value management, its principles, benefits, and helpful strategies to drive peak performance and cost-efficiency.