What is an ISO 14971 Checklist?
An ISO 14971 checklist is a form based on the guidelines of ISO 14971, a voluntary international standard that details how to apply risk management practices for medical devices. This ISO standard was developed by different experts in medicine, engineering, and quality management.
Why Use an ISO 14971 Checklist
While ISO 14971 is a voluntary standard, conforming to it ensures that good manufacturing processes are followed in the creation and risk management of medical devices. However, ISO 14971 is a nine-part standard, and keeping up with it can be challenging. For this reason, it can be beneficial to have a dedicated ISO 14971 checklist.
Having an ISO 14971 checklist can help streamline the workflows of medical device manufacturers, as it can assist them in better identifying which framework to use for product analysis, evaluation, review, and control. An ISO 14971 checklist can also help quality-check each stage or phase of a medical device’s life cycle, as it can help in analyzing risks related to data and system security, electricity management, radiation, biocompatibility, and overall usability.
An ISO 14971 checklist can also help with:
- Identifying issues with risk management and assessment
- Complying with internal standards and other ISO standards, such as ISO 13485, which details the creation and distribution of medical devices and related services
- Ensuring all proper procedures for creating and disposing of medical devices and related parts are followed
- Following risk management guidelines, such as that of ISO 31000
What to Include in an ISO 14971 Checklist
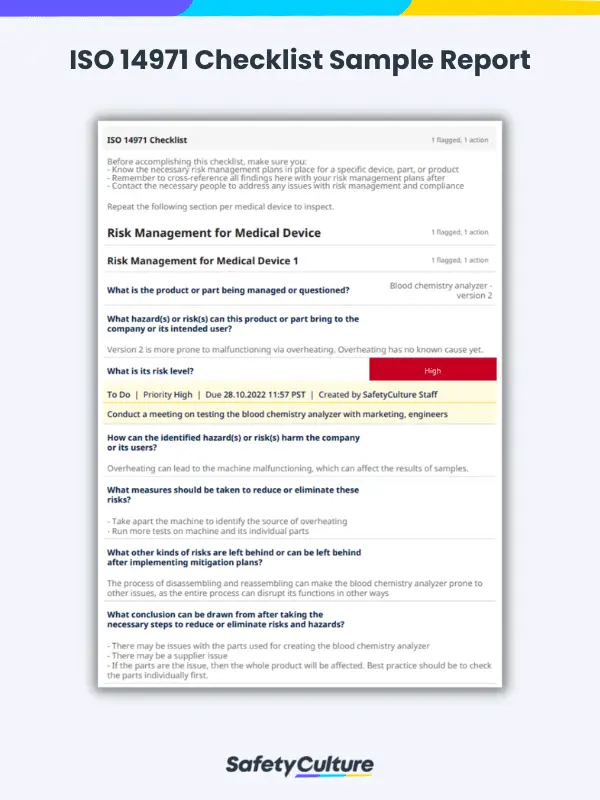
ISO 14971 Checklist Sample Report | SafetyCulture
Before anything else, a risk management plan should be set in place for all employees to follow. Each medical device and its parts should have its own risk management plan to guide employees on how to manage risks at every stage of its life cycle. The risk management plan should also detail what levels of risks are acceptable or manageable and should be put together in a Risk Management File (RMF) for easier access later on. The ISO 14971 checklist will then be used in conjunction with the risk management plans in place.
There are many ways to create an ISO 14971 checklist, as each manufacturer has different procedures, standards, and guidelines. However, a simple ISO 14971 checklist will ideally contain sections for the following:
- Description of the product: What is the product or part being managed or questioned?
- Risk analysis: What hazard(s) or risk(s) can this product or part bring to the company or its intended user?
- Risk evaluation: How can the identified hazard(s) or risk(s) harm the company or its users?
- Risk control or mitigation: What measures should be taken to reduce or eliminate risks?
- Evaluation of overall residual risk: What other kinds of risks are left behind or can be left behind after implementing mitigation plans?
- Risk management review: What conclusion can be drawn after taking the necessary steps to reduce or eliminate risks and hazards?
- Post-production review: Are there any recommendations for future risk management plans, uses, and the like?
After conducting a risk management inspection, the ISO 14971 checklist should be cross-referenced with the risk management plans. This is to identify other possible risks and problems in manufacturing, as well as ensure that all proper plans were followed.
FAQs about ISO 14971 Checklists
Manufacturers of medical devices are the primary audience for the ISO 14971 standard. However, some medical device users and sellers also refer to it as a basis for following good manufacturing procedures and standards.
An ISO 14971 checklist should be updated whenever there are changes in the medical device design, manufacturing process, or other relevant factors that may impact the process of risk management. And although there are no changes to the way the products were manufactured, it is ideal to have a regular review of the checklist to make sure that it is up-to-date and can effectively manage risks associated with medical device designs.
Using an ISO 14971 checklist helps in systematically evaluating and implementing the requirements of ISO 14971, ensuring that all necessary steps in the risk management process are followed and no crucial area will be missed.
Yes, ISO 14971 is a harmonized standard. A harmonized standard means that it is accepted by the European Commission and is compliant with the European Directives’ standards and requirements for use.