Published 10 Oct 2025
Article by
3 min read
What are ISO 17025 standards?
ISO/IEC 17025:2017 is the international standard that sets the requirements for the competency of laboratories in testing and calibration. Getting certified for the ISO/IEC 17025:2017 standard means that a laboratory is recognized to be technically proficient in testing and calibration and is performing at par with other internationally accredited peers in the industry. This certification also helps facilitate easier acceptance of test results and minimizes or eliminates the need for retesting.
What is an ISO 17025 Checklist?
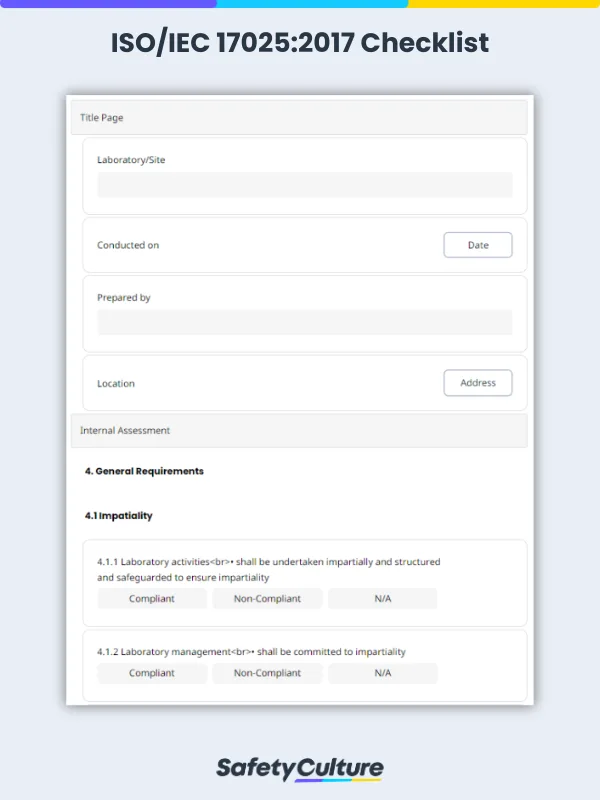
An ISO 17025:2017 checklist is a tool used by laboratory managers or metrologists to determine if a laboratory meets the required competencies for testing and calibration set by the ISO 17025:2017 standard. It contains the 5 main sections of the standard’s requirements: general, structural, resource, process, and management system requirements.
What is the difference between ISO 17025 and ISO 9001?
ISO 17025 and ISO 9001 are both quality management systems. But what sets them apart is the scope and the requirements. The requirements for ISO 17025 are specific to testing and calibration laboratories only. Meanwhile, ISO 9001 applies to organizations in all industries.
Clauses Covered in an ISO 17025 Checklist
An ISO/IEC 17025 checklist is built around the key clauses of the standard that testing and calibration laboratories must meet to demonstrate technical competence and consistent operations. What are the main sections or clauses covered in an ISO 17025 checklist?
Scope – Defines what types of testing and calibration activities are covered.
Normative references – Lists related standards and documents.
Terms and definitions – Clarifies important terminology.
Clause 4: General requirements – Focuses on impartiality and confidentiality in laboratory activities.
Clause 5: Structural requirements – Defines roles, responsibilities, and organizational structure to ensure accountability.
Clause 6: Resource requirements – Covers personnel competence, facilities, environmental conditions, equipment, and traceability of measurement standards.
Clause 7: Process requirements – The largest section, detailing operational procedures:
Review of requests, tenders, and contracts
Selection and verification of methods
Handling of test/calibration items
Technical records and measurement uncertainty
Ensuring validity of results (through QC, proficiency testing, etc.)
Reporting of results
Handling complaints, nonconforming work, and corrective actions
Control of data and information management systems
Clause 8: Management system requirements – Addresses quality management systems, documentation, control of records, internal audits, management reviews, and continual improvement.
How to Prepare for an ISO 17025 Certification
To ensure that a laboratory is prepared for ISO 17025:2017 certification, a coordinated effort within the organization is required. Here are 5 steps to follow in preparation for third-party certification:
Be familiar with the ISO 17025:2017 standard ; if the organization chooses option B for the standard’s management system requirement, be sure to review the ISO 9001 standard as well.
Conduct internal audits to determine gaps and discover areas for improvement.
Analyze the audit results and use the information collected to train employees and align processes with the standard.
Document the audit results, changes in the processes, and laboratory and employee competencies.
Contact a recognized third-party auditor and apply for ISO 17025 certification.

Thermosash was able to achieve ISO certification in six months using the SafetyCulture platform by centralizing workflows, boosting visibility, enhancing accountability, and streamlining quality control through QA checks and automatic corrective action alerts.