Published 20 Feb 2026
Article by
3 min read
What Is a CCP Validation Template?
A CCP validation template is a structured tool used in food safety systems to check if critical control points (CCPs) in your HACCP system are effectively keeping food safe. It logs evidence that compliance with CCP limits and requirements successfully prevents bacterial growth and other food safety hazards.
Why a CCP Validation Template Is Important
A CCP validation template scientifically demonstrates that your HACCP plan effectively stops hazards at key steps of the food handling process, ensuring food safety and compliance. Using this tool also:
Ensures hazard control works: It confirms that CCPs (such as cooking and chilling) meet safe limits, supported by tests and studies, to prevent bacterial growth. This reduces the risk of foodborne illnesses , minimizes waste, and ensures successful health inspection checks.
Helps teams meet legal and audit needs: The Food and Drug Administration’s (FDA) Hazard Analysis Critical Control Point (HACCP) Principles require validation of a strong food safety plan. For this, CCP validation templates may provide supporting documentation, especially during inspection checks.
Builds proof for training and corrective actions: The template tracks monitoring, spots failures early, and trains staff on fixes (e.g., retraining cooks if temperatures dip). It creates a solid audit trail, proving due diligence and providing ongoing tweaks to keep things safe.
Required Sections and Data Fields in a CCP Validation Template
A CCP validation template should include specific sections and fields to document proof that your CCPs in the food handling process can keep food safe according to HACCP standards. Below are the CCP validation examples that must be covered according to their respective sections.
Core Identification Fields
CCP name/description (e.g., “Cooking poultry”)
Hazard controlled (e.g., “Salmonella growth”)
Critical limits (e.g., "165°F for 15 seconds")
Scientific Evidence Section
Scientific support (e.g., FDA tables or peer-reviewed papers)
Process authority/expert input like signed letters from credible individuals
In-plant data, including your own logs or challenge studies
Monitoring and Verification Fields
Monitoring procedures (e.g., using a thermometer on every batch)
Frequency
Verification activities (e.g., through audits, calibrations, or tests)
Records/charts
Results and Action Fields
Validation results pass/fail summary
Corrective actions
Revalidation date
Signatures with date signed
How to Use a CCP Validation Template
Fill CCP basics: Write the CCP name, hazard, and safe limits. List how you'll monitor it, like thermometer checks for every batch.
Add proof data: Attach studies or FDA tables showing that the recommended critical limits work, along with your test logs from a few runs. Snap photos of temperatures or lab results if using a digital tool.
Record checks: Log who checks the CCP, when it was checked, and what the daily results are for a week. Note any misses and identify any patterns, like the oven running too cool on busy nights.
Review and sign off: Decide the pass/fail status based on recorded results. List corresponding fixes if needed (e.g., retrain staff). Get the manager to sign and date the document, and set a recheck date (e.g., yearly, monthly, etc.).
File and update: Save the template digitally for inspections. Don’t forget to revisit the document if recipes or equipment change. Training managers can also use the template to show real examples during staff training.
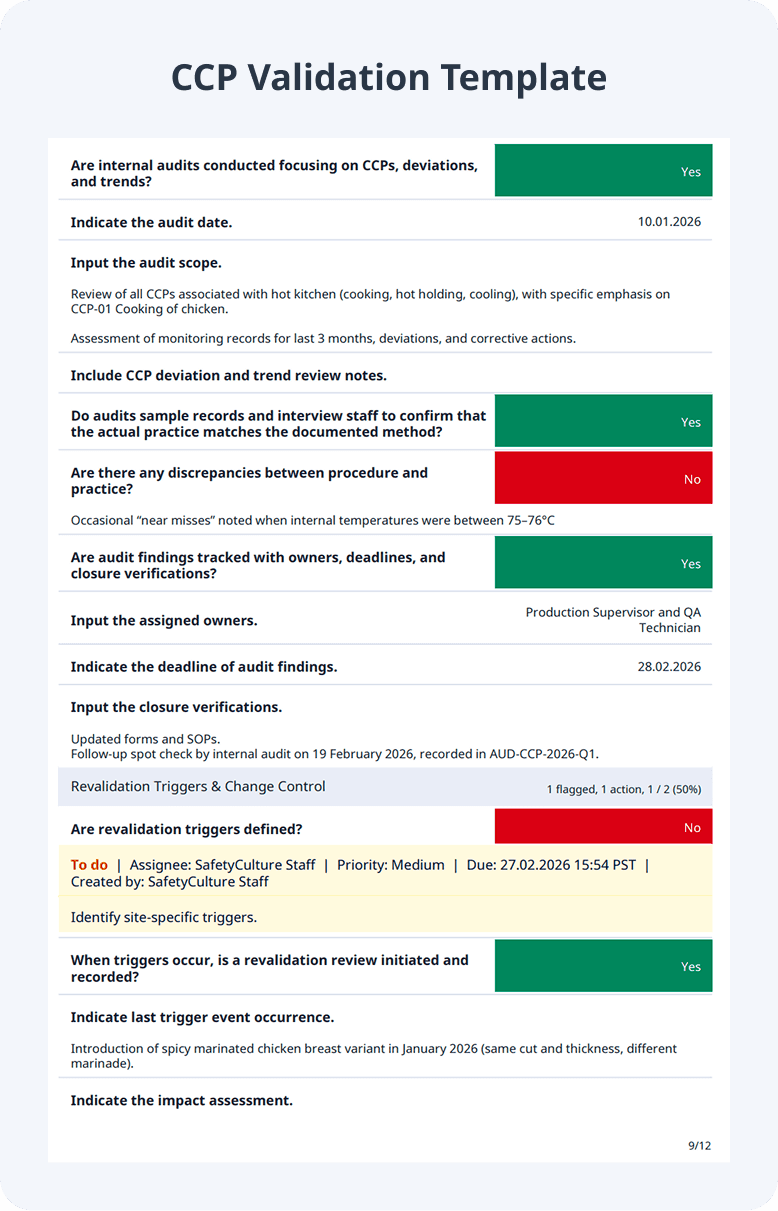
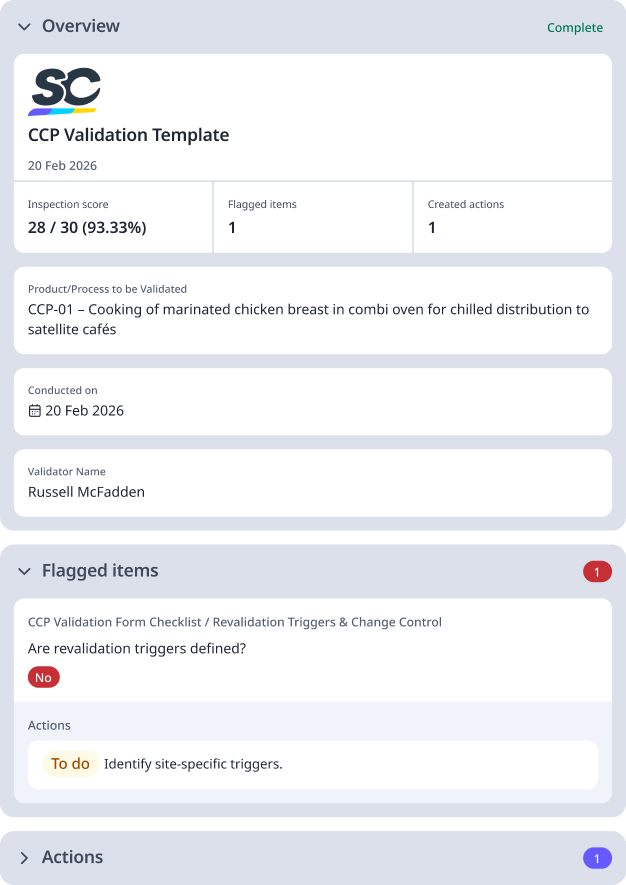
Sample CCP Validation Report
Below shows an example of a CCP validation report: